Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni
- PMID: 16237025
- PMCID: PMC1272998
- DOI: 10.1128/JB.187.21.7417-7424.2005
Bile salts modulate expression of the CmeABC multidrug efflux pump in Campylobacter jejuni
Abstract
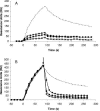
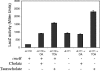
CmeABC, a multidrug efflux pump, is involved in the resistance of Campylobacter jejuni to a broad spectrum of antimicrobial agents and is essential for Campylobacter colonization in animal intestine by mediating bile resistance. Previously, we have shown that expression of this efflux pump is under the control of a transcriptional repressor named CmeR. Inactivation of CmeR or mutation in the cmeABC promoter (PcmeABC) region derepresses cmeABC, leading to overexpression of this efflux pump. However, it is unknown if the expression of cmeABC can be conditionally induced by the substrates it extrudes. In this study, we examined the expression of cmeABC in the presence of various antimicrobial compounds. Although the majority of the antimicrobials tested did not affect the expression of cmeABC, bile salts drastically elevated the expression of this efflux operon. The induction was observed with both conjugated and unconjugated bile salts and was in a dose- and time-dependent manner. Experiments using surface plasmon resonance demonstrated that bile salts inhibited the binding of CmeR to PcmeABC, suggesting that bile compounds are inducing ligands of CmeR. The interaction between bile salts and CmeR likely triggers conformational changes in CmeR, resulting in reduced binding affinity of CmeR to PcmeABC. Bile did not affect the transcription of cmeR, indicating that altered expression of cmeR is not a factor in bile-induced overexpression of cmeABC. In addition to the CmeR-dependent induction, some bile salts (e.g., taurocholate) also activated the expression of cmeABC by a CmeR-independent pathway. Consistent with the elevated production of CmeABC, the presence of bile salts in culture media resulted in increased resistance of Campylobacter to multiple antimicrobials. These findings reveal a new mechanism that modulates the expression of cmeABC and further support the notion that bile resistance is a natural function of CmeABC.
Figures
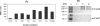
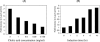
Similar articles
-
Regulation of the expression of the CmeABC efflux pump in Campylobacter jejuni: identification of a point mutation abolishing the binding of the CmeR repressor in an in vitro-selected multidrug-resistant mutant.FEMS Microbiol Lett. 2007 Feb;267(1):89-94. doi: 10.1111/j.1574-6968.2006.00558.x. Epub 2006 Dec 8. FEMS Microbiol Lett. 2007. PMID: 17166222
-
CmeR-dependent gene Cj0561c is induced more effectively by bile salts than the CmeABC efflux pump in both human and poultry Campylobacter jejuni strains.Res Microbiol. 2011 Dec;162(10):991-8. doi: 10.1016/j.resmic.2011.08.001. Epub 2011 Aug 31. Res Microbiol. 2011. PMID: 21920435
-
CmeR functions as a transcriptional repressor for the multidrug efflux pump CmeABC in Campylobacter jejuni.Antimicrob Agents Chemother. 2005 Mar;49(3):1067-75. doi: 10.1128/AAC.49.3.1067-1075.2005. Antimicrob Agents Chemother. 2005. PMID: 15728904 Free PMC article.
-
Structures of AcrR and CmeR: insight into the mechanisms of transcriptional repression and multi-drug recognition in the TetR family of regulators.Biochim Biophys Acta. 2009 May;1794(5):844-51. doi: 10.1016/j.bbapap.2008.12.001. Epub 2008 Dec 14. Biochim Biophys Acta. 2009. PMID: 19130905 Free PMC article. Review.
-
Antimicrobial Resistance in Campylobacter spp.Microbiol Spectr. 2018 Apr;6(2):10.1128/microbiolspec.arba-0013-2017. doi: 10.1128/microbiolspec.ARBA-0013-2017. Microbiol Spectr. 2018. PMID: 29623873 Free PMC article. Review.
Cited by
-
Selective depletion of Campylobacter jejuni via T6SS dependent functionality: an approach for improving chickens gut health.Gut Pathog. 2024 Jul 12;16(1):38. doi: 10.1186/s13099-024-00628-6. Gut Pathog. 2024. PMID: 38997758 Free PMC article.
-
Discovering novel bile protection systems in Bifidobacterium breve UCC2003 through functional genomics.Appl Environ Microbiol. 2012 Feb;78(4):1123-31. doi: 10.1128/AEM.06060-11. Epub 2011 Dec 9. Appl Environ Microbiol. 2012. PMID: 22156415 Free PMC article.
-
Campylobacter jejuni in Poultry: Pathogenesis and Control Strategies.Microorganisms. 2022 Oct 28;10(11):2134. doi: 10.3390/microorganisms10112134. Microorganisms. 2022. PMID: 36363726 Free PMC article. Review.
-
Akkermansia and its metabolites play key roles in the treatment of campylobacteriosis in mice.Front Immunol. 2023 Jan 12;13:1061627. doi: 10.3389/fimmu.2022.1061627. eCollection 2022. Front Immunol. 2023. PMID: 36713373 Free PMC article.
-
Salicylate functions as an efflux pump inducer and promotes the emergence of fluoroquinolone-resistant Campylobacter jejuni mutants.Appl Environ Microbiol. 2011 Oct;77(20):7128-33. doi: 10.1128/AEM.00763-11. Epub 2011 Aug 5. Appl Environ Microbiol. 2011. PMID: 21821741 Free PMC article.
References
-
- Black, R. E., M. M. Levine, M. L. Clements, T. P. Hughes, and M. J. Blaser. 1988. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 157:472-479. - PubMed
-
- Bron, P. A., M. Marco, S. M. Hoffer, E. Van Mullekom, W. M. de Vos, and M. Kleerebezem. 2004. Genetic characterization of the bile salt response in Lactobacillus plantarum and analysis of responsive promoters in vitro and in situ in the gastrointestinal tract. J. Bacteriol. 186:7829-7835. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

