Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR
- PMID: 16237072
- PMCID: PMC1473968
- DOI: 10.4049/jimmunol.175.9.5799
Recognition of fresh human tumor by human peripheral blood lymphocytes transduced with a bicistronic retroviral vector encoding a murine anti-p53 TCR
Erratum in
- J Immunol. 2006 Oct 15;177(8):5746
Abstract
The p53 protein is markedly up-regulated in a high proportion of human malignancies. Using an HLA-A2 transgenic mouse model, it was possible to isolate high-avidity murine CTLs that recognize class I-restricted human p53 epitopes. We isolated the alpha- and beta-chain of a TCR from a highly avid murine CTL clone that recognized the human p53(264-272) epitope. These genes were cloned into a retroviral vector that mediated high efficiency gene transfer into primary human lymphocytes. Efficiencies of >90% for gene transfer into lymphocytes were obtained without selection for transduced cells. The p53 TCR-transduced lymphocytes were able to specifically recognize with high-avidity, peptide-pulsed APCs as well as HLA-A2.1+ cells transfected with either wild-type or mutant p53 protein. p53 TCR-transduced cells demonstrated recognition and killing of a broad spectrum of human tumor cell lines as well as recognition of fresh human tumor cells. Interestingly, both CD8+ and CD4+ subsets were capable of recognizing and killing target cells, stressing the potential application of such a CD8-independent TCR molecule that can mediate both helper and cytotoxic responses. These results suggest that lymphocytes genetically engineered to express anti-p53 TCR may be of value for the adoptive immunotherapy of patients with a variety of common malignancies.
Conflict of interest statement
Disclosures
The authors have no financial conflict of interest.
Figures

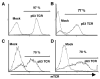







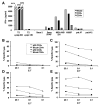
References
-
- Berzofsky, J. A., L. J. Helman, and D. P. Carbone. 2000. Cancer vaccines: cancer antigens, oncogenes and mutations. In Principles and Practices of the Biologic Therapy of Cancer. S. A. Rosenberg, ed. Lippincott Williams & Wilkins, Philadelphia, pp. 526–541.
-
- Levine AJ, Momand J, Finlay CA. The p53 tumour suppressor gene. Nature. 1991;351:453–456. - PubMed
-
- Olive KP, Tuveson DA, Ruhe ZC, Yin B, Willis NA, Bronson RT, Crowley D, Jacks T. Mutant p53 gain of function in two mouse models of Li-Fraumeni syndrome. Cell. 2004;119:847–860. - PubMed
-
- Lang GA, Iwakuma T, Suh YA, Liu G, Rao VA, Parant JM, Valentin-Vega YA, Terzian T, Caldwell LC, Strong LC, et al. Gain of function of a p53 hot spot mutation in a mouse model of Li-Fraumeni syndrome. Cell. 2004;119:861–872. - PubMed
-
- Winter SF, Minna JD, Johnson BE, Takahashi T, Gazdar AF, Carbone DP. Development of antibodies against p53 in lung cancer patients appears to be dependent on the type of p53 mutation. Cancer Res. 1992;52:4168–4174. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

