The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus
- PMID: 16237163
- PMCID: PMC6725736
- DOI: 10.1523/JNEUROSCI.2423-05.2005
The translation repressor 4E-BP2 is critical for eIF4F complex formation, synaptic plasticity, and memory in the hippocampus
Abstract
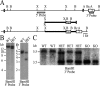
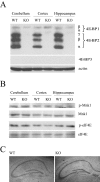
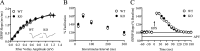
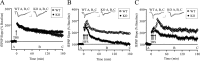
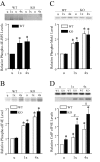
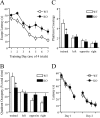
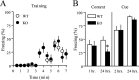
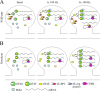
Long-lasting synaptic plasticity and memory requires mRNA translation, yet little is known as to how this process is regulated. To explore the role that the translation repressor 4E-BP2 plays in hippocampal long-term potentiation (LTP) and learning and memory, we examined 4E-BP2 knock-out mice. Interestingly, genetic elimination of 4E-BP2 converted early-phase LTP to late-phase LTP (L-LTP) in the Schaffer collateral pathway, likely as a result of increased eIF4F complex formation and translation initiation. A critical limit for activity-induced translation was revealed in the 4E-BP2 knock-out mice because L-LTP elicited by traditional stimulation paradigms was obstructed. Moreover, the 4E-BP2 knock-out mice also exhibited impaired spatial learning and memory and conditioned fear-associative memory deficits. These results suggest a crucial role for proper regulation of the eIF4F complex by 4E-BP2 during LTP and learning and memory in the mouse hippocampus.
Figures

References
-
- Abel T, Nguyen PV, Barad M, Deuel TA, Kandel ER, Bourtchouladze R (1997) Genetic demonstration of a role for PKA in the late phase of LTP and in hippocampus-based long-term memory. Cell 88: 615-626. - PubMed
-
- Banko JL, Hou L, Klann E (2004) NMDA receptor activation results in PKA- and ERK-dependent Mnk1 activation and increased eIF4E phosphorylation in hippocampal area CA1. J Neurochem 91: 462-470. - PubMed
-
- Barco A, Alarcon JM, Kandel ER (2002) Expression of constitutively active CREB protein facilitates the late phase of long-term potentiation by enhancing synaptic capture. Cell 108: 689-703. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
