Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease
- PMID: 16237164
- PMCID: PMC6725738
- DOI: 10.1523/JNEUROSCI.2546-05.2005
Blockade of nociceptin/orphanin FQ transmission attenuates symptoms and neurodegeneration associated with Parkinson's disease
Abstract
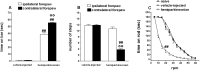
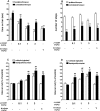
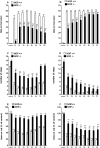
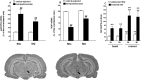
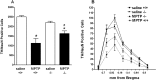
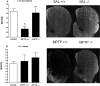
The opioid-like neuropeptide nociceptin/orphanin FQ (N/OFQ) and its receptor (NOP) are expressed in the substantia nigra (SN), a brain area containing dopamine neurons that degenerate in Parkinson's disease. Endogenous N/OFQ facilitates nigral glutamate release and inhibits nigrostriatal dopamine transmission and motor behavior. Here, we present evidence suggesting that endogenous N/OFQ may contribute to Parkinson's disease. Pharmacological blockade of the SN N/OFQ-NOP receptor system attenuated parkinsonian-like akinesia/hypokinesia in 6-hydroxydopamine hemilesioned or haloperidol-treated rats, whereas deletion of the NOP receptor gene conferred mice partial protection from haloperidol-induced motor depression. The antiparkinsonian action of NOP receptor antagonists was associated with reduction of glutamate release in the SN. In 6-hydroxydopamine hemilesioned rats, enhancement of N/OFQ expression and release was detected in the lesioned compared with the unlesioned SN, indicating that parkinsonism may be associated with overactivation of the N/OFQ-NOP receptor system in the SN. Finally, deletion of the N/OFQ gene conferred mice partial protection against 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced loss of SN dopamine neurons. Based on these data, we propose that NOP receptor antagonists may represent a novel approach for combined (symptomatic and neuroprotective) therapy of Parkinson's disease.
Figures



Similar articles
-
Managing Parkinson's disease: moving ON with NOP.Br J Pharmacol. 2020 Jan;177(1):28-47. doi: 10.1111/bph.14893. Epub 2020 Jan 3. Br J Pharmacol. 2020. PMID: 31648371 Free PMC article. Review.
-
Nociceptin/orphanin FQ receptor agonists attenuate L-DOPA-induced dyskinesias.J Neurosci. 2012 Nov 14;32(46):16106-19. doi: 10.1523/JNEUROSCI.6408-11.2012. J Neurosci. 2012. PMID: 23152595 Free PMC article.
-
Endogenous nociceptin/orphanin FQ (N/OFQ) contributes to haloperidol-induced changes of nigral amino acid transmission and parkinsonism: a combined microdialysis and behavioral study in naïve and nociceptin/orphanin FQ receptor knockout mice.Neuroscience. 2010 Mar 10;166(1):40-8. doi: 10.1016/j.neuroscience.2009.12.006. Epub 2009 Dec 13. Neuroscience. 2010. PMID: 20006677
-
Genetic and pharmacological evidence that endogenous nociceptin/orphanin FQ contributes to dopamine cell loss in Parkinson's disease.Neurobiol Dis. 2016 May;89:55-64. doi: 10.1016/j.nbd.2016.01.016. Epub 2016 Jan 22. Neurobiol Dis. 2016. PMID: 26804029 Free PMC article.
-
The biology of Nociceptin/Orphanin FQ (N/OFQ) related to obesity, stress, anxiety, mood, and drug dependence.Pharmacol Ther. 2014 Mar;141(3):283-99. doi: 10.1016/j.pharmthera.2013.10.011. Epub 2013 Nov 1. Pharmacol Ther. 2014. PMID: 24189487 Free PMC article. Review.
Cited by
-
NOP Receptor Ligands as Potential Agents for Inflammatory and Autoimmune Diseases.J Amino Acids. 2011;2011:836569. doi: 10.4061/2011/836569. Epub 2011 Nov 17. J Amino Acids. 2011. PMID: 22312472 Free PMC article.
-
Managing Parkinson's disease: moving ON with NOP.Br J Pharmacol. 2020 Jan;177(1):28-47. doi: 10.1111/bph.14893. Epub 2020 Jan 3. Br J Pharmacol. 2020. PMID: 31648371 Free PMC article. Review.
-
Activation of nociceptin/orphanin FQ peptide receptors disrupts visual but not auditory sensorimotor gating in BALB/cByJ mice: comparison to dopamine receptor agonists.Neuropsychopharmacology. 2012 Jan;37(2):378-89. doi: 10.1038/npp.2011.175. Epub 2011 Aug 31. Neuropsychopharmacology. 2012. PMID: 21881568 Free PMC article.
-
A Peptidomic Approach to Characterize Peptides Involved in Cerebellar Cortex Development Leads to the Identification of the Neurotrophic Effects of Nociceptin.Mol Cell Proteomics. 2018 Sep;17(9):1737-1749. doi: 10.1074/mcp.RA117.000184. Epub 2018 Jun 12. Mol Cell Proteomics. 2018. PMID: 29895708 Free PMC article.
-
Functional antagonism between nociceptin/orphanin FQ (N/OFQ) and corticotropin-releasing factor (CRF) in the rat brain: evidence for involvement of the bed nucleus of the stria terminalis.Psychopharmacology (Berl). 2008 Mar;196(4):523-31. doi: 10.1007/s00213-007-0985-7. Epub 2007 Nov 7. Psychopharmacology (Berl). 2008. PMID: 17989958
References
-
- Aparicio LC, Candeletti S, Binaschi A, Mazzuferi M, Mantovani S, Di Benedetto M, Landuzzi D, Lopetuso G, Romualdi P, Simonato M (2004) Kainate seizures increase nociceptin/orphanin FQ release in the rat hippocampus and thalamus: a microdialysis study. J Neurochem 91: 30-37. - PubMed
-
- Bergman H, Wichmann T, DeLong MR (1990) Reversal of experimental parkinsonism by lesions of the subthalamic nucleus. Science 249: 1436-1438. - PubMed
-
- Bertorelli R, Bastia E, Citterio F, Corradini L, Forlani A, Ongini E (2002) Lack of the nociceptin receptor does not affect acute or chronic nociception in mice. Peptides 23: 1589-1596. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
