Immunosuppression for liver transplantation in HCV-infected patients: mechanism-based principles
- PMID: 16237712
- PMCID: PMC2962573
- DOI: 10.1002/lt.20536
Immunosuppression for liver transplantation in HCV-infected patients: mechanism-based principles
Abstract
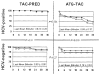
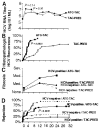
We retrospectively analyzed 42 hepatitis C virus (HCV)-infected patients who underwent cadaveric liver transplantation under two strategies of immunosuppression: (1) daily tacrolimus (TAC) throughout and an initial cycle of high-dose prednisone (PRED) with subsequent gradual steroid weaning, or (2) intraoperative antithymocyte globulin (ATG) and daily TAC that was later space weaned. After 36 +/- 4 months, patient and graft survival in the first group was 18/19 (94.7%) with no examples of clinically serious HCV recurrence. In the second group, the three-year patient survival was 12/23 (52%), and graft survival was 9/23 (39%); accelerated recurrent hepatitis was the principal cause of the poor results. The data were interpreted in the context of a recently proposed immunologic paradigm that is equally applicable to transplantation and viral immunity. In the framework of this paradigm, the disparate hepatitis outcomes reflected different equilibria reached under the two immunosuppression regimens between the relative kinetics of viral distribution (systemically and in the liver) and the slowly recovering HCV-specific T-cell response. As a corollary, the aims of treatment of the HCV-infected liver recipients should be to predict, monitor, and equilibrate beneficial balances between virus distribution and the absence of an immunopathologic antiviral T-cell response. In this view, favorable equilibria were accomplished in the nonweaned group of patients but not in the weaned group. In conclusion, since the anti-HCV response is unleashed when immunosuppression is weaned, treatment protocols that minimize disease recurrence in HCV-infected allograft recipients must balance the desire to reduce immunosuppression or induce allotolerance with the need to prevent antiviral immunopathology.
Figures

Comment in
-
The interrelation between recurrent hepatitis C, alloimmune response, and immunosuppression.Liver Transpl. 2005 Nov;11(11):1329-31. doi: 10.1002/lt.20588. Liver Transpl. 2005. PMID: 16237699 No abstract available.
References
-
- Kim WR, Poterucha JJ, Kremers WK, Ishitani MB, Dickson ER. Outcome of liver transplantation for hepatitis B in the United States. Liver Transpl. 2004;10:968–974. - PubMed
-
- Curry MP. Hepatitis B and hepatitis C viruses in liver transplantation. Transplantation. 2004;78:955–963. - PubMed
-
- Berenguer M, Crippin J, Gish R, Bass N, Bostrom A, Netto G, et al. A model to predict severe HCV-related disease following liver transplantation. Hepatology. 2003;38:34–41. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
