MaxiK channel partners: physiological impact
- PMID: 16239267
- PMCID: PMC1464300
- DOI: 10.1113/jphysiol.2005.098913
MaxiK channel partners: physiological impact
Abstract
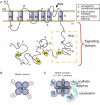
The basic functional unit of the large-conductance, voltage- and Ca2+-activated K+ (MaxiK, BK, BKCa) channel is a tetramer of the pore-forming alpha-subunit (MaxiKalpha) encoded by a single gene, Slo, holding multiple alternative exons. Depending on the tissue, MaxiKalpha can associate with modulatory beta-subunits (beta1-beta4) increasing its functional diversity. As MaxiK senses and regulates membrane voltage and intracellular Ca2+, it links cell excitability with cell signalling and metabolism. Thus, MaxiK is a key regulator of vital body functions, like blood flow, uresis, immunity and neurotransmission. Epilepsy with paroxysmal dyskinesia syndrome has been recognized as a MaxiKalpha-related disorder caused by a gain-of-function C-terminus mutation. This channel region is also emerging as a key recognition module containing sequences for MaxiKalpha interaction with its surrounding signalling partners, and its targeting to cell-specific microdomains. The growing list of interacting proteins highlights the possibility that associations with the C-terminus of MaxiKalpha are dynamic and depending on each cellular environment. We speculate that the molecular multiplicity of the C-terminus (and intracellular loops) dictated by alternative exons may modulate or create additional interacting sites in a tissue-specific manner. A challenge is the dissection of MaxiK macromolecular signalling complexes in different tissues and their temporal association/dissociation according to the stimulus.
Figures

References
-
- Benkusky NA, Fergus DJ, Zucchero TM, England SK. Regulation of the Ca2+-sensitive domains of the maxi-K channel in the mouse myometrium during gestation. J Biol Chem. 2000;275:27712–27719. - PubMed
-
- Brainard AM, Miller AJ, Martens JR, England SK. Maxi-K channels localize to caveolae in human myometrium: a role for an actin-channel-caveolin complex in the regulation of myometrial smooth muscle K+ current. Am J Physiol Cell Physiol. 2005;289:C49–C57. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

