Synaptic fatigue at the naive perforant path-dentate granule cell synapse in the rat
- PMID: 16239273
- PMCID: PMC1464272
- DOI: 10.1113/jphysiol.2005.097725
Synaptic fatigue at the naive perforant path-dentate granule cell synapse in the rat
Abstract
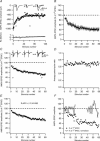
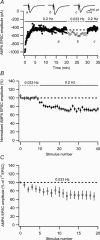
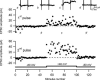
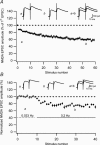
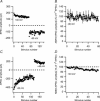
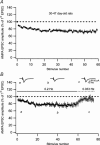
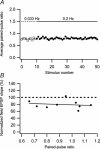
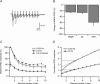
Synaptic activation at low frequency is often used to probe synaptic function and synaptic plasticity, but little is known about how such low-frequency activation itself affects synaptic transmission. In the present study, we have examined how the perforant path-dentate granule cell (PP-GC) synapse adapts to low-frequency activation from a previously non-activated (naive) state. Stimulation at 0.2 Hz in acute slices from developing rats (7-12 days old) caused a gradual depression of the AMPA EPSC (at -80 mV) to about half within 50 stimuli. This synaptic fatigue was unaffected by the NMDA and metabotropic glutamate (mGlu) receptor antagonists d-AP5 and LY-341495. A smaller component of this synaptic fatigue was readily reversible when switching to very low-frequency stimulation (0.033-0.017 Hz) and is attributed to a reversible decrease in release probability, which is probably due to depletion of readily releasable vesicles. Thus, it was expressed to the same extent by AMPA and NMDA EPSCs, and was associated with a decrease in quantal content (measured as 1/CV(2)) with no change in the paired-pulse ratio. The larger component of the synaptic fatigue was not readily reversible, was selective for AMPA EPSCs and was associated with a decrease in 1/CV(2), thus probably representing silencing of AMPA signalling in a subset of synapses. In adult rats (> 30 days old), the AMPA silencing had disappeared while the low-frequency depression remained unaltered. The present study has thus identified two forms of synaptic plasticity that contribute to fatigue of synaptic transmission at low frequencies at the developing PP-GC synapse; AMPA silencing and a low-frequency depression of release probability.
Figures

References
-
- Altman J, Das GD. Post-natal origin of microneurones in the rat brain. Nature. 1965;207:953–956. - PubMed
-
- Christoffersen GR. Habituation: events in the history of its characterization and linkage to synaptic depression. A new proposed kinetic criterion for its identification. Prog Neurobiol. 1997;53:45–66. - PubMed
-
- Clements JD. A statistical test for demonstrating a presynaptic site of action for a modulator of synaptic amplitude. J Neurosci Methods. 1990;31:75–88. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

