Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles
- PMID: 16239330
- PMCID: PMC1367066
- DOI: 10.1529/biophysj.105.061895
Photothermal nanotherapeutics and nanodiagnostics for selective killing of bacteria targeted with gold nanoparticles
Abstract
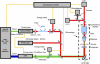
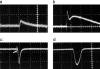
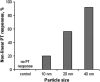
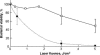
We describe a new method for selective laser killing of bacteria targeted with light-absorbing gold nanoparticles conjugated with specific antibodies. The multifunctional photothermal (PT) microscope/spectrometer provides a real-time assessment of this new therapeutic intervention. In this integrated system, strong laser-induced overheating effects accompanied by the bubble-formation phenomena around clustered gold nanoparticles are the main cause of bacterial damage. PT imaging and time-resolved monitoring of the integrated PT responses assessed these effects. Specifically, we used this technology for selective killing of the Gram-positive bacterium Staphylococcus aureus by targeting the bacterial surface using 10-, 20-, and 40-nm gold particles conjugated with anti-protein A antibodies. Labeled bacteria were irradiated with focused laser pulses (420-570 nm, 12 ns, 0.1-5 J/cm(2), 100 pulses), and laser-induced bacterial damage observed at different laser fluences and nanoparticle sizes was verified by optical transmission, electron microscopy, and conventional viability testing.
Figures





References
-
- Chopra, I. 2003. Antibiotic resistance in Staphylococcus aureus: concerns, causes and cures. Expert Rev. Anti Infect. Ther. 1:45–55. - PubMed
-
- Gemmell, G. C. 2004. Glycopeptide resistance in Staphylococcus aureus: is it a real threat? J. Infect. Chemother. 10:69–75. - PubMed
-
- Novick, R. P. 2003. Autoinduction and signal transduction in the regulation of staphylococcal virulence. Mol. Microbiol. 48:1429–1449. - PubMed
-
- Cheung, A. L., A. S. Bayer, G. Zhang, H. Gresham, and Y. Q. Xiong. 2004. Regulation of virulence determinants in vitro and in vivo in Staphylococcus aureus. FEMS Immunol. Med. Microsc. 40:1–9. - PubMed
-
- Moritz, A., O. Doertbudak, N. Gutknecht, K. Goharkhay, U. Schoop, and W. Sperr. 1997. Nd:YAG laser irradiation of infected root canals in combination with microbiological examinations. J. Am. Dent. Assoc. 128:1525–1530. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

