Shiga toxin 1 causes direct renal injury in rats
- PMID: 16239503
- PMCID: PMC1273854
- DOI: 10.1128/IAI.73.11.7099-7106.2005
Shiga toxin 1 causes direct renal injury in rats
Abstract
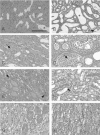
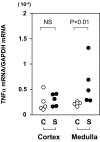
Infection with Shiga toxin (Stx)-producing Escherichia coli has been implicated to cause hemolytic uremic syndrome, which is characterized by histological abnormalities such as microvascular thrombi and tubular cell damage in the kidney. Although Stx is known to be the major virulence factor of the pathogen, it is still unclear whether Stx directly impairs renal cells in vivo to cause such histological changes and deterioration of renal function. To assess the consequence of the direct action of Stx on renal cells, left kidneys of rats were perfused with Stx1 from the renal artery through the renal vein and then revascularized. Kidneys of control animals were perfused with the vehicle alone. On day 1, apoptosis and induction of tumor necrosis factor alpha gene expression were noticed to occur in the medulla of the Stx1-perfused kidneys. On day 3, extensive tubular injuries were observed by light microscopy: aggregated platelets and monocytic infiltrates in both glomeruli and the medullary interstitium were detected by immunostaining. Tubular changes were more extensive on day 9, with areas of infarction seen in the cortex and medulla. These changes were not found to occur in the sham-operated kidneys. No obvious glomerular changes were detected by light microscopy at any time point. When nonperfused right kidneys were removed after the Stx1 perfusion of the left kidneys, the serum creatinine and blood urea nitrogen levels were increased from day 2, and acute renal failure followed on day 3. These results indicate that Stx1 caused glomerular platelet aggregation, tubular damage, and acute deterioration of renal function by acting directly on renal cells.
Figures





References
-
- Chomczynski, P., and N. Sacchi. 1987. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal. Biochem. 162:156-159. - PubMed
-
- Donnahoo, K. K., X. Meng, A. Ayala, M. P. Cain, A. H. Harken, and D. R. Meldrum. 1999. Early kidney TNF-α expression mediates neutrophil infiltration and injury after renal ischemia-reperfusion. Am. J. Physiol. 277:R922-R929. - PubMed
-
- Fenwick, B. W., and L. A. Cowan. 1998. Canine model of hemolytic-uremic syndrome, p. 268-277. In J. B. Kaper and A. D. O'Brien (ed.), Escherichia coli O157:H7 and other Shiga toxin-producing E. coli strains. ASM Press, Washington, D.C.
-
- Fitzpatrick, M. M., V. Shah, R. S. Trompeter, M. J. Dillon, and T. M. Barratt. 1992. Interleukin-8 and polymorphoneutrophil leucocyte activation in hemolytic uremic syndrome of childhood. Kidney Int. 42:951-956. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

