Disruption of tight junctions during traversal of the respiratory epithelium by Burkholderia cenocepacia
- PMID: 16239504
- PMCID: PMC1273860
- DOI: 10.1128/IAI.73.11.7107-7112.2005
Disruption of tight junctions during traversal of the respiratory epithelium by Burkholderia cenocepacia
Abstract
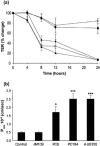
Burkholderia cenocepacia is an opportunistic bacterial species capable of causing life-threatening respiratory tract infection in persons with cystic fibrosis (CF). Unlike most other pathogens in CF, which typically remain confined to the endobronchial spaces, B. cenocepacia can traverse airway epithelium to cause bacteremia and sepsis. The mechanisms by which this occurs, however, are unknown. We examined the transmigration of B. cenocepacia through polarized respiratory epithelium. Representatives of three "epidemic" lineages common among CF patients in North America were able to traverse polarized 16HBE14o- cells in vitro. Transmigration of bacteria was associated with significant perturbations in epithelial permeability, as measured by a loss of transepithelial electrical resistance and increased flux of bovine serum albumin across the cell layer. Terminal deoxynucleotidyltransferase-mediated dUTP-biotin nick end labeling and trypan blue exclusion assays, as well as lactate dehydrogenase levels, did not indicate excessive cytotoxicity or cell death in infected cell layers. Rather, confocal fluorescence microscopy demonstrated the loss of occludin from tight junctions. In contrast, zonula occludens 1 was well preserved along intercellular borders. Western blot analysis showed a shift in the major occludin isoforms from high- to low-phosphorylation states during infection. These observations suggest that B. cenocepacia traverses polarized respiratory epithelium by the dephosphorylation and dissociation of occludin from the tight-junction complex.
Figures



References
-
- Aris, R. M., J. C. Routh, J. J. LiPuma, D. G. Heath, and P. H. Gilligan. 2001. Lung transplantation for cystic fibrosis patients with Burkholderia cepacia complex. Survival linked to genomovar type. Am. J. Respir. Crit. Care Med. 164:2102-2106. - PubMed
-
- Balda, M. S., and K. Matter. 1998. Tight junctions. J. Cell Sci. 111:541-547. - PubMed
-
- Balkovetz, D. F., and J. Katz. 2003. Bacterial invasion by a paracellular route: divide and conquer. Microbes Infect. 5:613-619. - PubMed
-
- Coenye, T., and J. J. LiPuma. 2003. Population structure analysis of Burkholderia cepacia genomovar III: varying degrees of genetic recombination characterize major clonal complexes. Microbiology 149:77-88. - PubMed
-
- Coenye, T., J. J. LiPuma, D. Henry, B. Hoste, K. Vandemeulebroecke, M. Gillis, D. P. Speert, and P. Vandamme. 2001. Burkholderia cepacia genomovar VI, a new member of the Burkholderia cepacia complex isolated from cystic fibrosis patients. Int. J. Syst. Evol. Microbiol. 51:271-279. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

