Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia
- PMID: 16239511
- PMCID: PMC1273880
- DOI: 10.1128/IAI.73.11.7170-7179.2005
Distinct CD4+-T-cell responses to live and heat-inactivated Aspergillus fumigatus conidia
Abstract
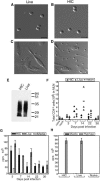
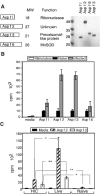
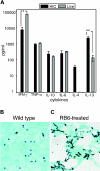
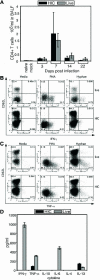
Aspergillus fumigatus is an important fungal pathogen that causes invasive pulmonary disease in immunocompromised hosts. Respiratory exposure to A. fumigatus spores also causes allergic bronchopulmonary aspergillosis, a Th2 CD4+-T-cell-mediated disease that accompanies asthma. The microbial factors that influence the differentiation of A. fumigatus-specific CD4+ T lymphocytes into Th1 versus Th2 cells remain incompletely defined. We therefore examined CD4+-T-cell responses of immunologically intact mice to intratracheal challenge with live or heat-inactivated A. fumigatus spores. Live but not heat-inactivated fungal spores resulted in recruitment of gamma interferon (IFN-gamma)-producing, fungus-specific CD4+ T cells to lung airways, achieving A. fumigatus-specific frequencies exceeding 5% of total CD4+ T cells. While heat-inactivated spores did not induce detectable levels of IFN-gamma-producing, A. fumigatus-specific CD4+ T cells in the airways, they did prime CD4+ T-cell responses in draining lymph nodes that produced greater amounts of interleukin 4 (IL-4) and IL-13 than T cells responding to live conidia. While immunization with live fungal spores induced antibody responses, we found a marked decrease in isotype-switched, A. fumigatus-specific antibodies in sera of mice following immunization with heat-inactivated spores. Our studies demonstrate that robust Th1 T-cell and humoral responses are restricted to challenge with fungal spores that have the potential to germinate and cause invasive infection. How the adaptive immune system distinguishes between metabolically active and inactive fungal spores remains an important question.
Figures

Similar articles
-
Innate immune activation and CD4+ T cell priming during respiratory fungal infection.Immunity. 2006 Oct;25(4):665-75. doi: 10.1016/j.immuni.2006.08.016. Epub 2006 Oct 5. Immunity. 2006. PMID: 17027299
-
Aspergillus fumigatus viability drives allergic responses to inhaled conidia.Ann Allergy Asthma Immunol. 2018 Aug;121(2):200-210.e2. doi: 10.1016/j.anai.2018.04.008. Epub 2018 Apr 13. Ann Allergy Asthma Immunol. 2018. PMID: 29660515
-
Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus.J Immunol. 2002 Feb 1;168(3):1362-71. doi: 10.4049/jimmunol.168.3.1362. J Immunol. 2002. PMID: 11801677
-
Immune responses to Aspergillus fumigatus infections.Biol Blood Marrow Transplant. 2006 Jan;12(1 Suppl 1):47-9. doi: 10.1016/j.bbmt.2005.09.007. Biol Blood Marrow Transplant. 2006. PMID: 16399584 Review.
-
The innate immune response to Aspergillus fumigatus at the alveolar surface.FEMS Microbiol Rev. 2015 Sep;39(5):670-87. doi: 10.1093/femsre/fuv018. Epub 2015 Apr 30. FEMS Microbiol Rev. 2015. PMID: 25934117 Review.
Cited by
-
Dectin-1 Promotes Type I and III Interferon Expression to Support Optimal Antifungal Immunity in the Lung.Front Cell Infect Microbiol. 2020 Jul 8;10:321. doi: 10.3389/fcimb.2020.00321. eCollection 2020. Front Cell Infect Microbiol. 2020. PMID: 32733815 Free PMC article.
-
Epidemiology of invasive mold infections in allogeneic stem cell transplant recipients: biological risk factors for infection according to time after transplantation.Clin Infect Dis. 2008 Oct 15;47(8):1041-50. doi: 10.1086/591969. Clin Infect Dis. 2008. PMID: 18781877 Free PMC article.
-
Aspergillus vaccines: Hardly worth studying or worthy of hard study?Med Mycol. 2017 Jan 1;55(1):103-108. doi: 10.1093/mmy/myw081. Epub 2016 Sep 17. Med Mycol. 2017. PMID: 27639242 Free PMC article. Review.
-
Type III interferon is a critical regulator of innate antifungal immunity.Sci Immunol. 2017 Oct 6;2(16):eaan5357. doi: 10.1126/sciimmunol.aan5357. Sci Immunol. 2017. PMID: 28986419 Free PMC article.
-
Microbial Components and Effector Molecules in T Helper Cell Differentiation and Function.Immune Netw. 2023 Feb 22;23(1):e7. doi: 10.4110/in.2023.23.e7. eCollection 2023 Feb. Immune Netw. 2023. PMID: 36911805 Free PMC article. Review.
References
-
- Bauman, S. K., K. L. Nichols, and J. W. Murphy. 2000. Dendritic cells in the induction of protective and nonprotective anticryptococcal cell-mediated immune responses. J. Immunol. 165:158-167. - PubMed
-
- Belosevic, M., D. S. Finbloom, P. H. Van Der Meide, M. V. Slayter, and C. A. Nacy. 1989. Administration of monoclonal anti-IFN-γ antibodies in vivo abrogates natural resistance of C3H/HeN mice to infection with Leishmania major. J. Immunol. 143:266-274. - PubMed
-
- Blease, K., C. Jakubzick, J. M. Schuh, B. H. Joshi, R. K. Puri, and C. M. Hogaboam. 2001. IL-13 fusion cytotoxin ameliorates chronic fungal-induced allergic airway disease in mice. J. Immunol. 167:6583-6592. - PubMed
-
- Blease, K., C. Jakubzick, J. Westwick, N. Lukacs, S. L. Kunkel, and C. M. Hogaboam. 2001. Therapeutic effect of IL-13 immunoneutralization during chronic experimental fungal asthma. J. Immunol. 166:5219-5224. - PubMed
-
- Bozza, S., R. Gaziano, A. Spreca, A. Bacci, C. Montagnoli, P. di Francesco, and L. Romani. 2002. Dendritic cells transport conidia and hyphae of Aspergillus fumigatus from the airways to the draining lymph nodes and initiate disparate Th responses to the fungus. J. Immunol. 168:1362-1371. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

