Characterization of two novel cryptococcal mannoproteins recognized by immune sera
- PMID: 16239533
- PMCID: PMC1273869
- DOI: 10.1128/IAI.73.11.7348-7355.2005
Characterization of two novel cryptococcal mannoproteins recognized by immune sera
Abstract
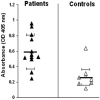
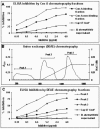
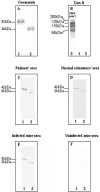
Host defenses against the encapsulated yeast Cryptococcus neoformans involve both humoral and cell-mediated immunity. Mannoproteins (MPs) are a heterogeneous class of immunodominant glycoproteins which have been only incompletely characterized. In this study, we report on the molecular features of two novel MPs that are recognized by serum antibodies during cryptococcosis. After fractionation of extracellular cryptococcal products, MPs reacted more strongly than other components with sera from C. neoformans-infected AIDS patients. Further fractionation and Western blot analysis of MPs evidenced the presence of highly reactive bands with molecular masses of 250, 125, 115, and 84 kDa. The 115- and 84-kDa bands contained significant amounts of N-linked oligosaccharides, as shown by decreased molecular mass after peptide-N-glycosidase F treatment. N-terminal amino acid sequences of the two bands were used to search C. neoformans nucleotide databases. Homologous genomic sequences were used to synthesize DNA probes and isolate cDNA clones containing the full-length genes, which were designated MP84 and MP115. Both genes showed the presence of a serine/threonine-rich region, a potential site for heavy glycosylation. MP84 and MP115 showed homology with, respectively, polysaccharide deacetylases and carboxylesterases from other organisms. Recombinant, deglycosylated proteins expressed in Escherichia coli still reacted with sera from patients, albeit more weakly than natural MPs, indicating that at least some of the reactive epitopes were retained in the recombinant forms. In conclusion, we identified two novel MPs that are important targets of antibody responses during cryptococcosis. These data may be useful to devise alternative immunity-based strategies to control the disease.
Figures



Similar articles
-
Molecular characterization of a mannoprotein with homology to chitin deacetylases that stimulates T cell responses to Cryptococcus neoformans.Proc Natl Acad Sci U S A. 2001 Aug 28;98(18):10422-7. doi: 10.1073/pnas.181331398. Epub 2001 Aug 14. Proc Natl Acad Sci U S A. 2001. PMID: 11504924 Free PMC article.
-
Purification and characterization of a second immunoreactive mannoprotein from Cryptococcus neoformans that stimulates T-Cell responses.Infect Immun. 2002 Oct;70(10):5485-93. doi: 10.1128/IAI.70.10.5485-5493.2002. Infect Immun. 2002. PMID: 12228274 Free PMC article.
-
A proteomic-based approach for the identification of immunodominant Cryptococcus neoformans proteins.Proteomics. 2009 May;9(9):2578-88. doi: 10.1002/pmic.200800713. Proteomics. 2009. PMID: 19343717 Free PMC article.
-
Antibody-mediated effects against Cryptococcus neoformans: evidence for interdependency and collaboration between humoral and cellular immunity.Res Immunol. 1998 May-Jun;149(4-5):321-33; discussion 500-3. doi: 10.1016/s0923-2494(98)80756-6. Res Immunol. 1998. PMID: 9720950 Review. No abstract available.
-
[Recognition of Cryptococcus neoformans by Pattern Recognition Receptors and its Role in Host Defense to This Infection].Med Mycol J. 2017;58(3):J83-J90. doi: 10.3314/mmj.17.011. Med Mycol J. 2017. PMID: 28855484 Review. Japanese.
Cited by
-
Vaccine and immunotherapeutic approaches for the prevention of cryptococcosis: lessons learned from animal models.Front Microbiol. 2012 Aug 28;3:291. doi: 10.3389/fmicb.2012.00291. eCollection 2012. Front Microbiol. 2012. PMID: 22973262 Free PMC article.
-
A Predicted Mannoprotein Cmp1 Regulates Fungal Virulence in Cryptococcus neoformans.Pathogens. 2020 Oct 24;9(11):881. doi: 10.3390/pathogens9110881. Pathogens. 2020. PMID: 33114434 Free PMC article.
-
Fungal Inositol Pyrophosphate IP7 Is Crucial for Metabolic Adaptation to the Host Environment and Pathogenicity.mBio. 2015 Jun 2;6(3):e00531-15. doi: 10.1128/mBio.00531-15. mBio. 2015. PMID: 26037119 Free PMC article.
-
The Cryptococcus wall: A different wall for a unique lifestyle.PLoS Pathog. 2023 Feb 23;19(2):e1011141. doi: 10.1371/journal.ppat.1011141. eCollection 2023 Feb. PLoS Pathog. 2023. PMID: 36821541 Free PMC article. No abstract available.
-
Protective immunity against cryptococcus neoformans infection.Mcgill J Med. 2007 Jan;10(1):35-43. Mcgill J Med. 2007. PMID: 18523595 Free PMC article.
References
-
- Biondo, C., A. Midiri, L. Messina, F. Tomasello, G. Garufi, M. R. Catania, M. Bombaci, C. Beninati, G. Teti, and G. Mancuso. 2005. MyD88 and TLR2, but not TLR4, are required for host defense against Cryptococcus neoformans. Eur. J. Immunol. 35:870-878. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
LinkOut - more resources
Full Text Sources

