Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci
- PMID: 16239538
- PMCID: PMC1273834
- DOI: 10.1128/IAI.73.11.7390-7397.2005
Enhanced factor H binding to sialylated Gonococci is restricted to the sialylated lacto-N-neotetraose lipooligosaccharide species: implications for serum resistance and evidence for a bifunctional lipooligosaccharide sialyltransferase in Gonococci
Erratum in
- Infect Immun. 2006 Apr;74(4):2503
Abstract
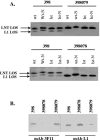
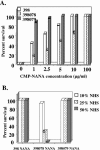
We isolated serologically identical (by serovar determination and porin variable region [VR] typing) strains of Neisseria gonorrhoeae from an infected male and two of his monogamous female sex partners. One strain (termed 398078) expressed the L1 (Galalpha1 --> 4 [corrected] Galbeta1 --> 4Glcbeta1 --> 4HepI) lipooligosaccharide (LOS) structure exclusively; the other (termed 398079) expressed the lacto-N-neotetraose (LNT; Galbeta1 --> 4GlcNAcbeta1 --> 3Galbeta1 --> 4Glcbeta1 --> 4HepI) LOS structure. The strain from the male index case expressed both glycoforms and exhibited both immunotypes. Nuclear magnetic resonance analysis revealed that sialic acid linked to the terminal Gal of L1 LOS via an alpha2 --> 6 linkage and, as expected, to the terminal Gal of LNT LOS via an alpha2--> 3 linkage. Insertional inactivation of the sialyltransferase gene (known to sialylate LNT LOS) abrogated both L1 LOS sialylation and LNT LOS sialylation, suggesting a bifunctional nature of this enzyme in gonococci. Akin to our previous observations, sialylation of the LNT LOS of strain 398079 enhanced the binding of the complement regulatory molecule, factor H. Rather surprisingly, factor H did not bind to sialylated strain 398078. LOS sialylation conferred the LNT LOS-bearing strain complete (100%) resistance to killing by even 50% nonimmune normal human serum (NHS), whereas sialylation of L1 LOS conferred resistance only to 10% NHS. The ability of gonococcal sialylated LNT to bind factor H confers high-level serum resistance, which is not seen with sialylated L1 LOS. Thus, serum resistance mediated by sialylation of gonococcal L1 and LNT LOS occurs by different mechanisms, and specificity of factor H binding to sialylated gonococci is restricted to the LNT LOS species.
Figures


References
-
- Campagnari, A. A., S. M. Spinola, A. J. Lesse, Y. A. Kwaik, R. E. Mandrell, and M. A. Apicella. 1990. Lipooligosaccharide epitopes shared among gram-negative non-enteric mucosal pathogens. Microb. Pathog. 8:353-362. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

