Defining the interaction of the Treponema pallidum adhesin Tp0751 with laminin
- PMID: 16239550
- PMCID: PMC1273862
- DOI: 10.1128/IAI.73.11.7485-7494.2005
Defining the interaction of the Treponema pallidum adhesin Tp0751 with laminin
Abstract
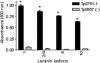
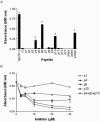
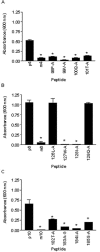
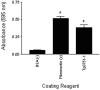
Various invasive pathogens attach to host tissues via the extracellular matrix component laminin, the major glycoprotein found within basement membranes. Previous investigations identified the laminin-binding adhesin Tp0751 within the spirochete bacterium Treponema pallidum. In the current study, Tp0751 was shown to attach to a variety of laminin isoforms that are widely distributed throughout the host, including laminins 1, 2, 4, 8, and 10. Such universal attachment is conducive for an adhesin present within a highly invasive pathogen that encounters a variety of tissue sites during the course of infection. Additional studies systematically identified the amino acid residues within Tp0751 that contribute to laminin binding using synthetic peptides designed from the mature protein sequence. The minimum laminin-binding region of the adhesin was localized to 10 amino acids; peptides containing these residues inhibited attachment of Tp0751 and T. pallidum to laminin. Further, Tp0751-specific antibodies inhibited attachment of T. pallidum to laminin. This study furthers our knowledge of the interaction of T. pallidum with laminin, an association that is proposed to facilitate bacterial traversal of basement membranes and subsequent entry into the circulation and tissue invasion. As such, these investigations will reveal new targets for possible prevention of bacterial dissemination and establishment of chronic infection.
Figures



Similar articles
-
Heterologous expression of the Treponema pallidum laminin-binding adhesin Tp0751 in the culturable spirochete Treponema phagedenis.J Bacteriol. 2008 Apr;190(7):2565-71. doi: 10.1128/JB.01537-07. Epub 2008 Feb 8. J Bacteriol. 2008. PMID: 18263731 Free PMC article.
-
Bifunctional role of the Treponema pallidum extracellular matrix binding adhesin Tp0751.Infect Immun. 2011 Mar;79(3):1386-98. doi: 10.1128/IAI.01083-10. Epub 2010 Dec 13. Infect Immun. 2011. PMID: 21149586 Free PMC article.
-
Identification of the Neuroinvasive Pathogen Host Target, LamR, as an Endothelial Receptor for the Treponema pallidum Adhesin Tp0751.mSphere. 2020 Apr 1;5(2):e00195-20. doi: 10.1128/mSphere.00195-20. mSphere. 2020. PMID: 32238570 Free PMC article.
-
Functional sites in the laminin alpha chains.Connect Tissue Res. 2005;46(3):142-52. doi: 10.1080/03008200591008527. Connect Tissue Res. 2005. PMID: 16147852 Review.
-
Laminins: structure and genetic regulation.Microsc Res Tech. 2000 Nov 1;51(3):214-27. doi: 10.1002/1097-0029(20001101)51:3<214::AID-JEMT2>3.0.CO;2-J. Microsc Res Tech. 2000. PMID: 11054872 Review.
Cited by
-
Laminin isoforms in development and disease.J Mol Med (Berl). 2007 Aug;85(8):825-36. doi: 10.1007/s00109-007-0182-5. Epub 2007 Apr 11. J Mol Med (Berl). 2007. PMID: 17426950 Review.
-
Syphilis.Nat Rev Dis Primers. 2017 Oct 12;3:17073. doi: 10.1038/nrdp.2017.73. Nat Rev Dis Primers. 2017. PMID: 29022569 Free PMC article. Review.
-
Treponema pallidum Lipoprotein TP0435 Expressed in Borrelia burgdorferi Produces Multiple Surface/Periplasmic Isoforms and mediates Adherence.Sci Rep. 2016 May 10;6:25593. doi: 10.1038/srep25593. Sci Rep. 2016. PMID: 27161310 Free PMC article.
-
The Borrelia burgdorferi outer-surface protein ErpX binds mammalian laminin.Microbiology (Reading). 2009 Mar;155(Pt 3):863-872. doi: 10.1099/mic.0.024604-0. Microbiology (Reading). 2009. PMID: 19246757 Free PMC article.
-
AMPlify: attentive deep learning model for discovery of novel antimicrobial peptides effective against WHO priority pathogens.BMC Genomics. 2022 Jan 25;23(1):77. doi: 10.1186/s12864-022-08310-4. BMC Genomics. 2022. PMID: 35078402 Free PMC article.
References
-
- Anonymous 2001. Venereal disease 2000. Institute of Medical Statistics CR. Institute of Medical Statistics, Prague, Czech Republic.
-
- Anonymous. 2004. Vancouver facing syphilis outbreak. AIDS Patient Care STDS 18:186. - PubMed
-
- Bruckner-Tuderman, L. 1999. Biology and pathology of the skin basement membrane zone. Matrix Biol. 18:3-4. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

