Murine macrophages kill the vegetative form of Bacillus anthracis
- PMID: 16239551
- PMCID: PMC1273904
- DOI: 10.1128/IAI.73.11.7495-7501.2005
Murine macrophages kill the vegetative form of Bacillus anthracis
Abstract
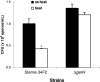
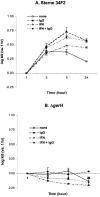
Anti-protective antigen antibody was reported to enhance macrophage killing of ingested Bacillus anthracis spores, but it was unclear whether the antibody-mediated macrophage killing mechanism was directed against the spore itself or the vegetative form emerging from the ingested and germinating spore. To address this question, we compared the killing of germination-proficient (gp) and germination-deficient (DeltagerH) Sterne 34F2 strain spores by murine peritoneal macrophages. While macrophages similarly ingested both spores, only gp Sterne was killed at 5 h (0.37 log kill). Pretreatment of macrophages with gamma interferon (IFN-gamma) or opsonization with immunoglobulin G (IgG) isolated from a subject immunized with an anthrax vaccine enhanced the killing of Sterne to 0.49 and 0.73 log, respectively, but the combination of IFN-gamma and IgG was no better than either treatment alone. Under no condition was there killing of DeltagerH spores. To examine the ability of the exosporium to protect spores from macrophages, we compared the macrophage-mediated killing of nonsonicated (exosporium+) and sonicated (exosporium-) Sterne 34F2 spores. More sonicated spores than nonsonicated spores were killed at 5 h (0.98 versus 0.37 log kill, respectively). Pretreatment with IFN-gamma increased the sonicated spore killing to 1.39 log. However, the opsonization with IgG was no better than no treatment or pretreatment with IFN-gamma. We conclude that macrophages appear unable to kill the spore form of B. anthracis and that the exosporium may play a role in the protection of spores from macrophages.
Figures
Similar articles
-
Recombinant Bacillus anthracis spore proteins enhance protection of mice primed with suboptimal amounts of protective antigen.Vaccine. 2008 Sep 8;26(38):4927-39. doi: 10.1016/j.vaccine.2008.07.015. Epub 2008 Jul 25. Vaccine. 2008. PMID: 18657585 Free PMC article.
-
The role of antibodies to Bacillus anthracis and anthrax toxin components in inhibiting the early stages of infection by anthrax spores.Microbiology (Reading). 2001 Jun;147(Pt 6):1677-1685. doi: 10.1099/00221287-147-6-1677. Microbiology (Reading). 2001. PMID: 11390699
-
A Bivalent Protein r-PAbxpB Comprising PA Domain IV and Exosporium Protein BxpB Confers Protection Against B. anthracis Spores and Toxin.Front Immunol. 2019 Mar 19;10:498. doi: 10.3389/fimmu.2019.00498. eCollection 2019. Front Immunol. 2019. PMID: 30941133 Free PMC article.
-
[Progress on the vaccine for anthrax].Wei Sheng Wu Xue Bao. 2005 Feb;45(1):149-52. Wei Sheng Wu Xue Bao. 2005. PMID: 15847185 Review. Chinese.
-
Glycan surface antigens from Bacillus anthracis as vaccine targets: current status and future perspectives.Expert Rev Vaccines. 2014 Jul;13(7):895-907. doi: 10.1586/14760584.2014.924404. Epub 2014 May 28. Expert Rev Vaccines. 2014. PMID: 24867680 Review.
Cited by
-
Murine aerosol challenge model of anthrax.Infect Immun. 2007 Jun;75(6):2689-98. doi: 10.1128/IAI.01875-06. Epub 2007 Mar 12. Infect Immun. 2007. PMID: 17353290 Free PMC article.
-
Recombinant exosporium protein BclA of Bacillus anthracis is effective as a booster for mice primed with suboptimal amounts of protective antigen.Infect Immun. 2007 Nov;75(11):5240-7. doi: 10.1128/IAI.00884-07. Epub 2007 Sep 4. Infect Immun. 2007. PMID: 17785478 Free PMC article.
-
Immunological alterations mediated by adenosine during host-microbial interactions.Immunol Res. 2011 May;50(1):69-77. doi: 10.1007/s12026-011-8207-0. Immunol Res. 2011. PMID: 21479929 Free PMC article. Review.
-
Proteins involved in formation of the outermost layer of Bacillus subtilis spores.J Bacteriol. 2011 Aug;193(16):4075-80. doi: 10.1128/JB.05310-11. Epub 2011 Jun 10. J Bacteriol. 2011. PMID: 21665972 Free PMC article.
-
Myeloid differentiation primary response gene 88 is required for the resolution of otitis media.J Infect Dis. 2008 Dec 15;198(12):1862-9. doi: 10.1086/593213. J Infect Dis. 2008. PMID: 18986247 Free PMC article.
References
-
- Baillie, L. 2001. The development of new vaccines against Bacillus anthracis. J. Appl. Bacteriol. 91:609-613. - PubMed
-
- Baillie, L., S. Hibbs, P. Tsai, G. L. Cao, and G. M. Rosen. 2005. Role of superoxide in the germination of Bacillus anthracis endospores. FEMS Microbiol. Lett. 245:33-38. - PubMed
-
- Baillie, L., T. Townend, N. Walker, U. Eriksson, and D. Williamson. 2004. Characterization of the human immune response to the UK anthrax vaccine. FEMS Immunol. Med. Microbiol. 42:267-270. - PubMed
-
- Barlass, P. J., C. W. Houston, M. O. Clements, and A. Moir. 2002. Germination of Bacillus cereus spores in response to l-alanine and to inosine: the roles of gerL and gerQ operons. Microbiology 148:2089-2095. - PubMed
-
- Dixon, T. C., A. A. Fadl, T. M. Koehler, J. A. Swanson, and P. C. Hanna. 2000. Early Bacillus anthracis-macrophage interactions: intracellular survival and escape. Cell. Microbiol. 2:453-463. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

