Metalloproteinase inhibitors, nonantimicrobial chemically modified tetracyclines, and ilomastat block Bacillus anthracis lethal factor activity in viable cells
- PMID: 16239558
- PMCID: PMC1273843
- DOI: 10.1128/IAI.73.11.7548-7557.2005
Metalloproteinase inhibitors, nonantimicrobial chemically modified tetracyclines, and ilomastat block Bacillus anthracis lethal factor activity in viable cells
Abstract
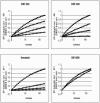
Lethal toxin, produced by the bacterium Bacillus anthracis, is a major contributor to morbidity and mortality in animals and humans who have contracted anthrax. One component of this toxin, lethal factor (LF), proteolytically inactivates members of the mitogen-activated protein kinase kinase (MAPKK or MEK) family. In this study we show that CMT-300, CMT-308, and Ilomastat, agents initially characterized as matrix metalloproteinase inhibitors which are in early stages of development as pharmaceuticals, effectively inhibit the zinc metalloproteinase activity of LF. All three inhibitors, CMT-300, CMT-308, and Ilomastat, inhibit LF-mediated cleavage of a synthetic peptide substrate based on the N-terminal domain of MEKs. Inhibition of LF-mediated MEK proteolysis by all three agents was also achieved using lysates of the human monocytoid line MonoMac 6 as sources of MAPKKs and visualization of the extent of cleavage after separation by sodium dodecyl sulfate-polyacrylamide gel electrophoresis followed by detection by Western blotting. Finally, we have demonstrated inhibition of intracellular MEKs in viable human monocytes and MonoMac 6 cells by these agents after incubation of the cells with a reconstituted preparation of recombinant lethal toxin. All three agents are effective inhibitors when incubated with LF prior to exposure to cells, while the CMTs, but not Ilomastat, are also effective when added after LF has already entered the viable cell targets. These results offer promise for strategies to combat effects of the lethal toxin of B. anthracis.
Figures

References
-
- Barletta, J. P., G. Angella, K. C. Balch, H. G. Dimova, G. A. Stern, M. T. Moser, G. B. van Setten, and G. S. Schultz. 1996. Inhibition of pseudomonal ulceration in rabbit corneas by a synthetic matrix metalloproteinase inhibitor. Investig. Ophthalmol. Vis. Sci. 37:20-28. - PubMed
-
- Bradley, K. A., J. Mogridge, M. Mourez, R. J. Collier, and J. A. T. Young. 2001. Identification of the cellular receptor for anthrax toxin. Nature 414:225-229. - PubMed
-
- Bradley, K. A., J. Mogridge, G. Jonah, A. Rainey, S. Batty, and J. A. Young. 2003. Binding of anthrax toxin to its receptor is similar to alpha integrin-ligand interactions. J. Biol. Chem. 278:49342-49347. - PubMed
-
- Brossier, F., and M. Mock. 2001. Toxins of Bacillus anthracis. Toxicon 39:1747-1755. - PubMed
-
- Carney, D. E., C. J. Lutz, A. L. Picone, L. A. Gatto, G. Bailey, M. Fillinger, and G. F. Neiman. 1999. Matrix metalloproteinase inhibitor prevents acute lung injury after cardiopulmonary bypass. Circulation 100:400-406. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

