Effects of Anaplasma phagocytophilum on host cell ferritin mRNA and protein levels
- PMID: 16239567
- PMCID: PMC1273867
- DOI: 10.1128/IAI.73.11.7629-7636.2005
Effects of Anaplasma phagocytophilum on host cell ferritin mRNA and protein levels
Abstract
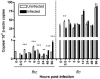
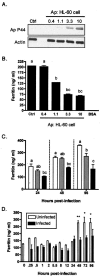
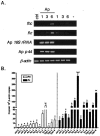
Ferritin is a major intracellular iron storage protein and also functions as a cytoprotectant by sequestering iron to minimize the formation of reactive oxygen species. Anaplasma phagocytophilum, the causative agent of human granulocytic anaplasmosis, is an obligate intracellular bacterium that colonizes neutrophils. We have previously reported that human promyelocytic HL-60 cells infected with A. phagocytophilum demonstrate increased transcription of ferritin heavy chain and also that the bacterium stimulates neutrophil NADPH oxidase assembly and degranulation during the initial hours of infection (J. A. Carlyon, W. T. Chan, J. Galan, D. Roos, and E. Fikrig, J. Immunol. 169:7009-7018, 2002, and J. A. Carlyon, D. Abdel-Latif, M. Pypaert, P. Lacy, and E. Fikrig, Infect. Immun. 72:4772-4783, 2004). In this study, we assessed ferritin mRNA and protein levels during A. phagocytophilum infection in vitro using HL-60 cells and neutrophils and in vivo using neutrophils from infected mice. The addition of A. phagocytophilum, as well as Escherichia coli and serum-opsonized zymosan, to neutrophils results in a pronounced increase in ferritin light-chain transcription and a concomitant rise in ferritin protein levels. Neutrophils from A. phagocytophilum-infected mice demonstrate elevated ferritin heavy-chain mRNA expression, a phenomenon consistent with infections by intracellular pathogens. Notably, ferritin protein levels of infected HL-60 cells were markedly diminished in a dose- and time-dependent manner. These studies provide insight into the effects A. phagocytophilum has on the ferritin levels of its host cell.
Figures
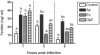

Similar articles
-
Anaplasma phagocytophilum utilizes multiple host evasion mechanisms to thwart NADPH oxidase-mediated killing during neutrophil infection.Infect Immun. 2004 Aug;72(8):4772-83. doi: 10.1128/IAI.72.8.4772-4783.2004. Infect Immun. 2004. PMID: 15271939 Free PMC article.
-
Anaplasma phagocytophilum-induced gene expression in both human neutrophils and HL-60 cells.Genomics. 2008 Sep;92(3):144-51. doi: 10.1016/j.ygeno.2008.05.005. Epub 2008 Jul 7. Genomics. 2008. PMID: 18603403
-
Laboratory Maintenance of Anaplasma phagocytophilum.Curr Protoc Microbiol. 2005 Jul;Chapter 3:Unit 3A.2. doi: 10.1002/9780471729259.mc03a02s00. Curr Protoc Microbiol. 2005. PMID: 18770564
-
Invasion and survival strategies of Anaplasma phagocytophilum.Cell Microbiol. 2003 Nov;5(11):743-54. doi: 10.1046/j.1462-5822.2003.00323.x. Cell Microbiol. 2003. PMID: 14531890 Review.
-
Human granulocytic anaplasmosis and Anaplasma phagocytophilum.Emerg Infect Dis. 2005 Dec;11(12):1828-34. doi: 10.3201/eid1112.050898. Emerg Infect Dis. 2005. PMID: 16485466 Free PMC article. Review.
Cited by
-
Evasion of host antioxidative response via disruption of NRF2 signaling in fatal Ehrlichia-induced liver injury.PLoS Pathog. 2023 Nov 13;19(11):e1011791. doi: 10.1371/journal.ppat.1011791. eCollection 2023 Nov. PLoS Pathog. 2023. PMID: 37956169 Free PMC article.
-
Severe Human Granulocytic Anaplasmosis With Significantly Elevated Ferritin Levels in an Immunocompetent Host in Pennsylvania: A Case Report.J Investig Med High Impact Case Rep. 2018 Feb 13;6:2324709618758350. doi: 10.1177/2324709618758350. eCollection 2018 Jan-Dec. J Investig Med High Impact Case Rep. 2018. PMID: 29468169 Free PMC article.
-
Ehrlichia chaffeensis TRP32 interacts with host cell targets that influence intracellular survival.Infect Immun. 2012 Jul;80(7):2297-306. doi: 10.1128/IAI.00154-12. Epub 2012 Apr 30. Infect Immun. 2012. PMID: 22547548 Free PMC article.
-
Anaplasma phagocytophilum APH_1387 is expressed throughout bacterial intracellular development and localizes to the pathogen-occupied vacuolar membrane.Infect Immun. 2010 May;78(5):1864-73. doi: 10.1128/IAI.01418-09. Epub 2010 Mar 8. Infect Immun. 2010. PMID: 20212090 Free PMC article.
-
Anaplasma phagocytophilum MSP2(P44)-18 predominates and is modified into multiple isoforms in human myeloid cells.Infect Immun. 2008 May;76(5):2090-8. doi: 10.1128/IAI.01594-07. Epub 2008 Feb 19. Infect Immun. 2008. PMID: 18285495 Free PMC article.
References
-
- Ahmed, N., J. F. Williams, and M. J. Weidemann. 1991. The human promyelocytic HL60 cell line: a model of myeloid cell differentiation using dimethylsulphoxide, phorbol ester and butyrate. Biochem. Int. 23:591-602. - PubMed
-
- Bakken, J. S., J. S. Dumler, S. M. Chen, M. R. Eckman, L. L. Van Etta, and D. H. Walker. 1994. Human granulocytic ehrlichiosis in the upper midwest United States. A new species emerging? JAMA 272:212-218. - PubMed
-
- Balla, G., H. S. Jacob, J. Balla, M. Rosenberg, K. Nath, F. Apple, J. W. Eaton, and G. M. Vercellotti. 1992. Ferritin: a cytoprotective antioxidant strategem of endothelium. J. Biol. Chem. 267:18148-18153. - PubMed
-
- Banerjee, R., J. Anguita, D. Roos, and E. Fikrig. 2000. Infection by the agent of human granulocytic ehrlichiosis prevents the respiratory burst by down-regulating gp91phox. J. Immunol. 164:3946-3949. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

