Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization
- PMID: 16239570
- PMCID: PMC1273872
- DOI: 10.1128/IAI.73.11.7657-7668.2005
Uropathogenic Escherichia coli flagella aid in efficient urinary tract colonization
Abstract
In the murine model of urinary tract infections (UTI), cystitis by uropathogenic Escherichia coli (UPEC) occurs through an intimate relationship with the bladder superficial umbrella cell entailing cycles of adherence, invasion, intracellular bacterial community (IBC) formation, and dispersal (fluxing) from the intracellular environment. IBC dispersal is a key step that results in the spread of bacteria over the epithelial surface to initiate additional rounds of IBC formation. We investigated the role of flagella in mediating adherence and motility during UTI, hypothesizing that the dispersion of the IBC would be incomplete in the absence of motility, thus interrupting the IBC pathway and attenuating the infection. Using gfp reporter fusions, the expression of the flagellar class I flhDC and class III fliC genes was monitored to track key points of regulation throughout the pathogenic cascade. In vitro, growth under conditions promoting motility resulted in the robust expression of both fusions. In contrast, only the class I fusion produced significant expression throughout early stages of IBC development including the dispersion stage. Thus, unlike in vitro modeling of motility, the regulatory cascade appeared incomplete in vivo. Throughout IBC formation, nonmotile DeltafliC mutants achieved the same number of IBCs as the wild-type (wt) strain, demonstrating that flagella are neither essential nor required for first- or second-generation IBC formation. However, in competition experiments between wt and DeltafliC strains, the wt was shown to have a fitness advantage in persisting throughout the urinary tract for 2 weeks, demonstrating a subtle but measurable role for flagella in virulence.
Figures
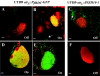
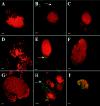
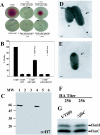
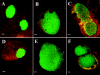

Similar articles
-
Expression of flagella is coincident with uropathogenic Escherichia coli ascension to the upper urinary tract.Proc Natl Acad Sci U S A. 2007 Oct 16;104(42):16669-74. doi: 10.1073/pnas.0607898104. Epub 2007 Oct 9. Proc Natl Acad Sci U S A. 2007. PMID: 17925449 Free PMC article.
-
Role of motility in the colonization of uropathogenic Escherichia coli in the urinary tract.Infect Immun. 2005 Nov;73(11):7644-56. doi: 10.1128/IAI.73.11.7644-7656.2005. Infect Immun. 2005. PMID: 16239569 Free PMC article.
-
Metabolic Requirements of Escherichia coli in Intracellular Bacterial Communities during Urinary Tract Infection Pathogenesis.mBio. 2016 Apr 12;7(2):e00104-16. doi: 10.1128/mBio.00104-16. mBio. 2016. PMID: 27073089 Free PMC article.
-
Bacterial characteristics of importance for recurrent urinary tract infections caused by Escherichia coli.Dan Med Bull. 2011 Apr;58(4):B4187. Dan Med Bull. 2011. PMID: 21466767 Review.
-
Covert operations of uropathogenic Escherichia coli within the urinary tract.Traffic. 2005 Jan;6(1):18-31. doi: 10.1111/j.1600-0854.2004.00251.x. Traffic. 2005. PMID: 15569242 Free PMC article. Review.
Cited by
-
Roles of OmpX, an Outer Membrane Protein, on Virulence and Flagellar Expression in Uropathogenic Escherichia coli.Infect Immun. 2021 May 17;89(6):e00721-20. doi: 10.1128/IAI.00721-20. Print 2021 May 17. Infect Immun. 2021. PMID: 33753414 Free PMC article.
-
The Ferric Citrate Uptake System Encoded in a Novel bla CTX-M-3- and bla TEM-1-Harboring Conjugative Plasmid Contributes to the Virulence of Escherichia coli.Front Microbiol. 2021 May 26;12:667782. doi: 10.3389/fmicb.2021.667782. eCollection 2021. Front Microbiol. 2021. PMID: 34122381 Free PMC article.
-
Gardnerella Exposures Alter Bladder Gene Expression and Augment Uropathogenic Escherichia coli Urinary Tract Infection in Mice.Front Cell Infect Microbiol. 2022 Jun 16;12:909799. doi: 10.3389/fcimb.2022.909799. eCollection 2022. Front Cell Infect Microbiol. 2022. PMID: 35782131 Free PMC article.
-
Trade-off between bile resistance and nutritional competence drives Escherichia coli diversification in the mouse gut.PLoS Genet. 2011 Jun;7(6):e1002107. doi: 10.1371/journal.pgen.1002107. Epub 2011 Jun 16. PLoS Genet. 2011. PMID: 21698140 Free PMC article.
-
Multiple genes repress motility in uropathogenic Escherichia coli constitutively expressing type 1 fimbriae.J Bacteriol. 2008 May;190(10):3747-56. doi: 10.1128/JB.01870-07. Epub 2008 Mar 21. J Bacteriol. 2008. PMID: 18359812 Free PMC article.
References
-
- Anderson, G. G., J. J. Palermo, J. D. Schilling, R. Roth, J. Heuser, and S. J. Hultgren. 2003. Intracellular bacterial biofilm-like pods in urinary tract infections. Science 301:105-107. - PubMed
-
- Bahrani-Mougeot, F. K., E. L. Buckles, C. V. Lockatell, J. R. Hebel, D. E. Johnson, C. M. Tang, and M. S. Donnenberg. 2002. Type 1 fimbriae and extracellular polysaccharides are preeminent uropathogenic Escherichia coli virulence determinants in the murine urinary tract. Mol. Microbiol. 45: 1079-1093. - PubMed
-
- Berin, M. C., A. Darfeuille-Michaud, L. J. Egan, Y. Miyamoto, and M. F. Kagnoff. 2002. Role of EHEC O157:H7 virulence factors in the activation of intestinal epithelial cell NF-κB and MAP kinase pathways and the upregulated expression of interleukin 8. Cell. Microbiol. 4:635-648. - PubMed
-
- Burall, L. S., J. M. Harro, X. Li, C. V. Lockatell, S. D. Himpsl, J. R. Hebel, D. E. Johnson, and H. L. Mobley. 2004. Proteus mirabilis genes that contribute to pathogenesis of urinary tract infection: identification of 25 signature-tagged mutants attenuated at least 100-fold. Infect. Immun. 72:2922-2938. - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

