Exposure to mycobacteria primes the immune system for evolutionarily diverse heat shock proteins
- PMID: 16239573
- PMCID: PMC1273840
- DOI: 10.1128/IAI.73.11.7687-7696.2005
Exposure to mycobacteria primes the immune system for evolutionarily diverse heat shock proteins
Abstract
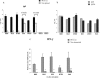
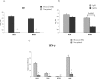
During stress conditions, such as infection, the synthesis of heat shock proteins (HSPs) in microorganisms is upregulated. Since a high degree of homology exists within each HSP family, we postulated that exposure to microorganisms could prime the immune system for evolutionarily diverse HSPs. We tested this hypothesis by priming mice with three microorganisms, namely, Mycobacterium bovis BCG, Mycobacterium vaccae, and Chlamydia pneumoniae. After this, mice received a dose of the various HSPs. We found that BCG and M. vaccae but not C. pneumoniae primed the immune system for the induction of secondary immunoglobulin G (IgG) responses to most of the HSPs tested. Analysis of the IgG1 and IgG2a profile and gamma interferon production induced against the HSPs revealed the induction of a mixture of responses. We also observed that sera from mice treated with M. vaccae and HSP70 were cross-reactive, but no antibody complexes were observed in their kidneys, which frequently are targets for autoantibody reactions. Our findings add further support for the use of HSPs as effective vaccine adjuvants.
Figures
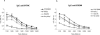
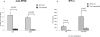

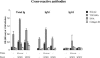
Similar articles
-
Mycobacterial heat-shock proteins as carrier molecules. II: The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette Guérin priming.Eur J Immunol. 1992 Jun;22(6):1365-72. doi: 10.1002/eji.1830220606. Eur J Immunol. 1992. PMID: 1601031
-
Endothelial cytotoxicity mediated by serum antibodies to heat shock proteins of Escherichia coli and Chlamydia pneumoniae: immune reactions to heat shock proteins as a possible link between infection and atherosclerosis.Circulation. 1999 Mar 30;99(12):1560-6. doi: 10.1161/01.cir.99.12.1560. Circulation. 1999. PMID: 10096931
-
Mycobacterial heat-shock proteins as carrier molecules.Eur J Immunol. 1991 Oct;21(10):2297-302. doi: 10.1002/eji.1830211002. Eur J Immunol. 1991. PMID: 1680693
-
Heat shock (stress) proteins and autoimmunity in rheumatic diseases.Semin Arthritis Rheum. 1993 Jun;22(6):357-74. doi: 10.1016/s0049-0172(05)80028-9. Semin Arthritis Rheum. 1993. PMID: 8342043 Review.
-
Are heat shock proteins involved in autoimmunity?Int J Clin Lab Res. 1992;22(2):90-4. doi: 10.1007/BF02591403. Int J Clin Lab Res. 1992. PMID: 1504311 Review.
Cited by
-
Mycobacteria-Based Vaccines as Immunotherapy for Non-urological Cancers.Cancers (Basel). 2020 Jul 5;12(7):1802. doi: 10.3390/cancers12071802. Cancers (Basel). 2020. PMID: 32635668 Free PMC article. Review.
-
Chlamydial Hsp60-2 is iron responsive in Chlamydia trachomatis serovar E-infected human endometrial epithelial cells in vitro.Infect Immun. 2007 May;75(5):2374-80. doi: 10.1128/IAI.01465-06. Epub 2007 Feb 16. Infect Immun. 2007. PMID: 17307941 Free PMC article.
-
Genomic analysis of Mycobacterium brumae sustains its nonpathogenic and immunogenic phenotype.Front Microbiol. 2023 Jan 5;13:982679. doi: 10.3389/fmicb.2022.982679. eCollection 2022. Front Microbiol. 2023. PMID: 36687580 Free PMC article.
-
The Distinct Role of Small Heat Shock Protein 20 on HCV NS3 Expression in HEK-293T Cell Line.Avicenna J Med Biotechnol. 2018 Jul-Sep;10(3):152-157. Avicenna J Med Biotechnol. 2018. PMID: 30090208 Free PMC article.
References
-
- Ahlborg, N., F. Sterky, D. Haddad, P. Perlmann, P. Å. Nygren, R. Andersson, and K. Berzins. 1997. Predominance of H-2d- and H-2k-restricted T-cell epitopes in the highly repetitive Plasmodium falciparum antigen Pf332. Mol. Immunol. 34:379-389. - PubMed
-
- al-Balaghi, S., E. Moller, G. Moller, and M. Abedi-Valugerdi. 1996. Mercury induces polyclonal B cell activation, autoantibody production and renal immune complex deposits in young (NZB x NZW)F1 hybrids. Eur. J. Immunol. 26:1519-1526. - PubMed
-
- Barrios, C., A. R. Lussow, J. Van Embden, R. Van der Zee, R. Rappuoli, P. Costantino, J. A. Louis, P. H. Lambert, and G. Del Giudice. 1992. Mycobacterial heat-shock proteins as carrier molecules. II. The use of the 70-kDa mycobacterial heat-shock protein as carrier for conjugated vaccines can circumvent the need for adjuvants and Bacillus Calmette-Guérin priming. Eur. J. Immunol. 22:1365-1372. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

