Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes
- PMID: 16239964
- PMCID: PMC1257537
- DOI: 10.1172/JCI25105
Lewis X component in human milk binds DC-SIGN and inhibits HIV-1 transfer to CD4+ T lymphocytes
Abstract
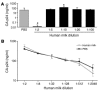
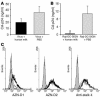
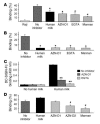
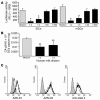
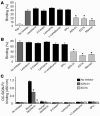
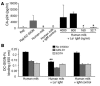
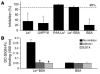
DC-specific ICAM3-grabbing non-integrin (DC-SIGN), which is expressed on DCs, can interact with a variety of pathogens such as HIV-1, hepatitis C, Ebola, cytomegalovirus, Dengue virus, Mycobacterium, Leishmania, and Candida albicans. We demonstrate that human milk can inhibit the DC-SIGN-mediated transfer of HIV-1 to CD4+ T lymphocytes as well as viral transfer by both immature and mature DCs. The inhibitory factor directly interacted with DC-SIGN and prevented the HIV-1 gp120 envelope protein from binding to the receptor. The human milk proteins lactoferrin, alpha-lactalbumin, lysozyme, beta-casein, and secretory leukocyte protease inhibitor did not bind DC-SIGN or demonstrate inhibition of viral transfer. The inhibitory effect could be fully alleviated with an Ab recognizing the Lewis X (LeX) sugar epitope, commonly found in human milk. LeX in polymeric form or conjugated to protein could mimic the inhibitory activity, whereas free LeX sugar epitopes could not. We reveal that a LeX motif present in human milk can bind to DC-SIGN and thereby prevent the capture and subsequent transfer of HIV-1 to CD4+ T lymphocytes. The presence of such a DC-SIGN-binding molecule in human milk may both influence antigenic presentation and interfere with pathogen transfer in breastfed infants.
Figures

Similar articles
-
Bile salt-stimulated lipase from human milk binds DC-SIGN and inhibits human immunodeficiency virus type 1 transfer to CD4+ T cells.Antimicrob Agents Chemother. 2006 Oct;50(10):3367-74. doi: 10.1128/AAC.00593-06. Antimicrob Agents Chemother. 2006. PMID: 17005819 Free PMC article.
-
Mucin 6 in seminal plasma binds DC-SIGN and potently blocks dendritic cell mediated transfer of HIV-1 to CD4(+) T-lymphocytes.Virology. 2009 Sep 1;391(2):203-11. doi: 10.1016/j.virol.2009.06.011. Virology. 2009. PMID: 19682628
-
MUC1 in human milk blocks transmission of human immunodeficiency virus from dendritic cells to T cells.Mol Immunol. 2009 Jul;46(11-12):2309-16. doi: 10.1016/j.molimm.2009.03.025. Epub 2009 Apr 29. Mol Immunol. 2009. PMID: 19406479
-
DC-SIGN points the way to a novel mechanism for HIV-1 transmission.MedGenMed. 2003 May 23;5(2):2. MedGenMed. 2003. PMID: 14603101 Review.
-
DC-SIGN (dendritic cell-specific ICAM-grabbing non-integrin) and DC-SIGN-related (DC-SIGNR): friend or foe?Clin Sci (Lond). 2003 Apr;104(4):437-46. Clin Sci (Lond). 2003. PMID: 12653690 Review.
Cited by
-
Profiles of human milk oligosaccharides and production of some human milk oligosaccharides in transgenic animals.Adv Nutr. 2012 May 1;3(3):456S-64S. doi: 10.3945/an.111.001529. Adv Nutr. 2012. PMID: 22585925 Free PMC article. Review.
-
Dietary Supplementation with Nondigestible Oligosaccharides Reduces Allergic Symptoms and Supports Low Dose Oral Immunotherapy in a Peanut Allergy Mouse Model.Mol Nutr Food Res. 2018 Oct;62(20):e1800369. doi: 10.1002/mnfr.201800369. Epub 2018 Aug 31. Mol Nutr Food Res. 2018. PMID: 30102006 Free PMC article.
-
Identification of the optimal DC-SIGN binding site on human immunodeficiency virus type 1 gp120.J Virol. 2007 Aug;81(15):8325-36. doi: 10.1128/JVI.01765-06. Epub 2007 May 23. J Virol. 2007. PMID: 17522223 Free PMC article.
-
Molecular Recognition in C-Type Lectins: The Cases of DC-SIGN, Langerin, MGL, and L-Sectin.Chembiochem. 2020 Nov 2;21(21):2999-3025. doi: 10.1002/cbic.202000238. Epub 2020 Jul 2. Chembiochem. 2020. PMID: 32426893 Free PMC article. Review.
-
Efficient Chemoenzymatic Synthesis of an N-glycan Isomer Library.Chem Sci. 2015 Oct 1;6(10):5652-5661. doi: 10.1039/C5SC02025E. Epub 2015 Jun 23. Chem Sci. 2015. PMID: 26417422 Free PMC article.
References
-
- Mitchell DA, Fadden AJ, Drickamer K. A novel mechanism of carbohydrate recognition by the C-type lectins DC-SIGN and DC-SIGNR. Subunit organization and binding to multivalent ligands. J. Biol. Chem. 2001;276:28939–28945. - PubMed
-
- Weis WI, Taylor ME, Drickamer K. The C-type lectin superfamily in the immune system. Immunol. Rev. 1998;163:19–34. - PubMed
-
- Geijtenbeek TB, et al. DC-SIGN, a dendritic cell-specific HIV-1-binding protein that enhances trans-infection of T cells. Cell. 2000;100:587–597. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

