Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction
- PMID: 16239966
- PMCID: PMC1257535
- DOI: 10.1172/JCI24912
Hemin-activated macrophages home to the pancreas and protect from acute pancreatitis via heme oxygenase-1 induction
Abstract
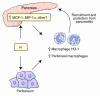
Hemin upregulates heme oxygenase-1 (HO-1), a stress-induced enzyme implicated in protection from a variety of injuries while its related isoform HO-2 is constitutively expressed. The role of hemin or HO-1 in the pancreas and their potential modulation of pancreatic injury are unknown. We show that HO-1 is induced in pancreatitis caused by caerulein and more prominently in severe pancreatitis caused by feeding a choline-deficient diet (CDD). Intraperitoneal hemin administration dramatically increases peritoneal and pancreas macrophages that overexpress HO-1 in association with pancreatic induction of the chemoattractants monocyte chemotactic protein-1 and macrophage inflammatory protein-1alpha but not RANTES or macrophage inflammatory protein-2. Hemin administration before CDD feeding protected 8 of 8 mice from lethality while 7 of 16 controls died. Protection is mediated by HO-1-overexpressing macrophages since hemin-primed macrophages home to the pancreas after transfer to naive mice and protect from CDD-induced pancreatitis. Suppression of hemin-primed peritoneal cell HO-1 using HO-1-specific small interfering RNA prior to cell transfer abolishes protection from CDD-induced pancreatitis. Similarly, hemin pretreatment in caerulein-induced pancreatitis reduces serum amylase and lipase, decreases pancreatic trypsin generation, and protects from lung injury. Therefore, hemin-like compounds or hemin-activated macrophages may offer novel therapeutic approaches for preventing acute pancreatitis and its pulmonary complication via upregulation of HO-1.
Figures

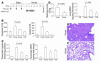




Similar articles
-
Induction of heme oxygenase-1 with hemin reduces obesity-induced adipose tissue inflammation via adipose macrophage phenotype switching.Mediators Inflamm. 2014;2014:290708. doi: 10.1155/2014/290708. Epub 2014 Nov 11. Mediators Inflamm. 2014. PMID: 25477711 Free PMC article.
-
Protective effects of heme oxygenase-1 against severe acute pancreatitis via inhibition of tumor necrosis factor-α and augmentation of interleukin-10.BMC Gastroenterol. 2017 Aug 24;17(1):100. doi: 10.1186/s12876-017-0651-4. BMC Gastroenterol. 2017. PMID: 28836936 Free PMC article.
-
Panhematin provides a therapeutic benefit in experimental pancreatitis.Gut. 2011 May;60(5):671-9. doi: 10.1136/gut.2010.217208. Epub 2010 Dec 15. Gut. 2011. PMID: 21159893 Free PMC article.
-
Heme oxygenase-1 in environmental toxin-induced lung disease.Toxicol Mech Methods. 2012 Jun;22(5):323-9. doi: 10.3109/15376516.2012.666685. Epub 2012 Mar 21. Toxicol Mech Methods. 2012. PMID: 22394342 Review.
-
Macrophages in the pancreas: Villains by circumstances, not necessarily by actions.Immun Inflamm Dis. 2020 Dec;8(4):807-824. doi: 10.1002/iid3.345. Epub 2020 Sep 3. Immun Inflamm Dis. 2020. PMID: 32885589 Free PMC article. Review.
Cited by
-
Heme oxygenase, inflammation, and fibrosis: the good, the bad, and the ugly?Front Pharmacol. 2012 May 7;3:81. doi: 10.3389/fphar.2012.00081. eCollection 2012. Front Pharmacol. 2012. PMID: 22586396 Free PMC article.
-
Tricetin Reduces Inflammation and Acinar Cell Injury in Cerulein-Induced Acute Pancreatitis: The Role of Oxidative Stress-Induced DNA Damage Signaling.Biomedicines. 2022 Jun 10;10(6):1371. doi: 10.3390/biomedicines10061371. Biomedicines. 2022. PMID: 35740393 Free PMC article.
-
Single-Cell Transcriptomic Analysis of the Mouse Pancreas: Characteristic Features of Pancreatic Ductal Cells in Chronic Pancreatitis.Genes (Basel). 2022 Jun 5;13(6):1015. doi: 10.3390/genes13061015. Genes (Basel). 2022. PMID: 35741777 Free PMC article.
-
Possible mechanisms mediating the protective effect of cilostazol in L-arginine induced acute pancreatitis in rats: role of cGMP, cAMP, and HO-1.Naunyn Schmiedebergs Arch Pharmacol. 2020 Oct;393(10):1859-1870. doi: 10.1007/s00210-020-01897-z. Epub 2020 May 19. Naunyn Schmiedebergs Arch Pharmacol. 2020. PMID: 32424476
-
Acute pancreatitis in aging animals: loss of pancreatitis-associated protein protection?World J Gastroenterol. 2012 Jul 14;18(26):3379-88. doi: 10.3748/wjg.v18.i26.3379. World J Gastroenterol. 2012. PMID: 22807607 Free PMC article.
References
-
- Soares MP, et al. Expression of heme oxygenase-1 can determine cardiac xenograft survival. Nat. Med. 1998;4:1073–1077. - PubMed
-
- Lee TS, Chau LY. Heme oxygenase-1 mediates the anti-inflammatory effect of interleukin-10 in mice. Nat. Med. 2002;8:240–246. - PubMed
-
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. - PubMed
-
- Durante W. Heme oxygenase-1 in growth control and its clinical application to vascular disease. J. Cell. Physiol. 2003;195:373–382. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

