Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides
- PMID: 16239967
- PMCID: PMC1257534
- DOI: 10.1172/JCI24792
Treatment of murine Th1- and Th2-mediated inflammatory bowel disease with NF-kappa B decoy oligonucleotides
Abstract
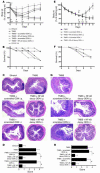
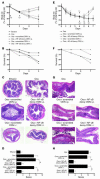
The Th1 and Th2 T cell responses that underlie inflammatory bowel diseases (IBDs) are likely to depend on NF-kappaB transcriptional activity. We explored this possibility in studies in which we determined the capacity of NF-kappaB decoy oligodeoxynucleotides (decoy ODNs) to treat various murine models of IBD. In initial studies, we showed that i.r. (intrarectal) or i.p. administration of decoy ODNs encapsulated in a viral envelope prevented and treated a model of acute trinitrobenzene sulfonic acid-induced (TNBS-induced) colitis, as assessed by clinical course and effect on Th1 cytokine production. In further studies, we showed that NF-kappaB decoy ODNs were also an effective treatment of a model of chronic TNBS-colitis, inhibiting both the production of IL-23/IL-17 and the development of fibrosis that characterizes this model. Treatment of TNBS-induced inflammation by i.r. administration of NF-kappaB decoy ODNs did not inhibit NF-kappaB in extraintestinal organs and resulted in CD4+ T cell apoptosis, suggesting that such treatment is highly focused and durable. Finally, we showed that NF-kappaB decoy ODNs also prevented and treated oxazolone-colitis and thus affect a Th2-mediated inflammatory process. In each case, decoy administration led to inflammation-clearing effects, suggesting a therapeutic potency applicable to human IBD.
Figures






References
-
- Podolsky DK. Inflammatory bowel disease. N. Engl. J. Med. 2002;347:417–429. - PubMed
-
- Bouma G, Strober W. The immunological and genetic basis of inflammatory bowel disease. Nat. Rev. Immunol. 2003;3:521–533. - PubMed
-
- Sartor RB. Therapeutic manipulation of the enteric microflora in inflammatory bowel diseases: antibiotics, probiotics, and prebiotics. Gastroenterology. 2004;126:1620–1633. - PubMed
-
- Maeda S, et al. Nod2 mutation in Crohn’s disease potentiates NF-κB activity and IL-1β processing. Science. 2005;307:734–738. - PubMed
-
- Heller F, Fuss IJ, Nieuwenhuis EE, Blumberg RS, Strober W. Oxazolone colitis, a Th2 colitis model resembling ulcerative colitis, is mediated by IL-13-producing NK-T cells. Immunity. 2002;17:629–638. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

