Coupling of protein motions and hydrogen transfer during catalysis by Escherichia coli dihydrofolate reductase
- PMID: 16241906
- PMCID: PMC1386024
- DOI: 10.1042/BJ20051464
Coupling of protein motions and hydrogen transfer during catalysis by Escherichia coli dihydrofolate reductase
Abstract
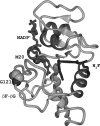
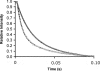
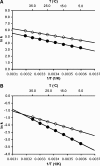
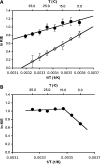
The enzyme DHFR (dihydrofolate reductase) catalyses hydride transfer from NADPH to, and protonation of, dihydrofolate. The physical basis of the hydride transfer step catalysed by DHFR from Escherichia coli has been studied through the measurement of the temperature dependence of the reaction rates and the kinetic isotope effects. Single turnover experiments at pH 7.0 revealed a strong dependence of the reaction rates on temperature. The observed relatively large difference in the activation energies for hydrogen and deuterium transfer led to a temperature dependence of the primary kinetic isotope effects from 3.0+/-0.2 at 5 degrees C to 2.2+/-0.2 at 40 degrees C and an inverse ratio of the pre-exponential factors of 0.108+/-0.04. These results are consistent with theoretical models for hydrogen transfer that include contributions from quantum mechanical tunnelling coupled with protein motions that actively modulate the tunnelling distance. Previous work had suggested a coupling of a remote residue,Gly121, with the kinetic events at the active site. However, pre-steady-state experiments at pH 7.0 with the mutant G121V-DHFR, in which Gly121 was replaced with valine, revealed that the chemical mechanism of DHFR catalysis was robust to this replacement. The reduced catalytic efficiency of G121V-DHFR was mainly a consequence of the significantly reduced pre-exponential factors, indicating the requirement for significant molecular reorganization during G121V-DHFR catalysis. In contrast, steady-state measurements at pH 9.5, where hydride transfer is rate limiting, revealed temperature-independent kinetic isotope effects between 15 and 35 degrees C and a ratio of the pre-exponential factors above the semi-classical limit, suggesting a rigid active site configuration from which hydrogen tunnelling occurs. The mechanism by which hydrogen tunnelling in DHFR is coupled with the environment appears therefore to be sensitive to pH.
Figures
Similar articles
-
Solvent effects on catalysis by Escherichia coli dihydrofolate reductase.J Am Chem Soc. 2010 Jan 27;132(3):1137-43. doi: 10.1021/ja909353c. J Am Chem Soc. 2010. PMID: 20047317
-
Evidence for a functional role of the dynamics of glycine-121 of Escherichia coli dihydrofolate reductase obtained from kinetic analysis of a site-directed mutant.Biochemistry. 1997 Dec 16;36(50):15792-800. doi: 10.1021/bi9716231. Biochemistry. 1997. PMID: 9398309
-
Hydride transfer during catalysis by dihydrofolate reductase from Thermotoga maritima.Biochem J. 2003 Sep 1;374(Pt 2):529-35. doi: 10.1042/BJ20030412. Biochem J. 2003. PMID: 12765545 Free PMC article.
-
Probing coupled motions in enzymatic hydrogen tunnelling reactions.Biochem Soc Trans. 2009 Apr;37(Pt 2):349-53. doi: 10.1042/BST0370349. Biochem Soc Trans. 2009. PMID: 19290860 Review.
-
Searching sequence space: two different approaches to dihydrofolate reductase catalysis.Chembiochem. 2005 Apr;6(4):590-600. doi: 10.1002/cbic.200400237. Chembiochem. 2005. PMID: 15812782 Review.
Cited by
-
Thermal adaptation of dihydrofolate reductase from the moderate thermophile Geobacillus stearothermophilus.Biochemistry. 2014 May 6;53(17):2855-63. doi: 10.1021/bi500238q. Epub 2014 Apr 22. Biochemistry. 2014. PMID: 24730604 Free PMC article.
-
Evidence that a 'dynamic knockout' in Escherichia coli dihydrofolate reductase does not affect the chemical step of catalysis.Nat Chem. 2012 Mar 11;4(4):292-7. doi: 10.1038/nchem.1296. Nat Chem. 2012. PMID: 22437714
-
Temperature-Dependent Kinetic Isotope Effects in R67 Dihydrofolate Reductase from Path-Integral Simulations.J Phys Chem B. 2021 Feb 11;125(5):1369-1377. doi: 10.1021/acs.jpcb.0c10318. Epub 2021 Feb 1. J Phys Chem B. 2021. PMID: 33522797 Free PMC article.
-
An Analysis of All the Relevant Facts and Arguments Indicates that Enzyme Catalysis Does Not Involve Large Contributions from Nuclear Tunneling.J Phys Org Chem. 2010 Jul;23(7):677-684. doi: 10.1002/poc.1620. J Phys Org Chem. 2010. PMID: 21494414 Free PMC article. No abstract available.
-
Carbon-deuterium bonds as probes of dihydrofolate reductase.J Am Chem Soc. 2008 May 21;130(20):6597-603. doi: 10.1021/ja0779607. Epub 2008 Apr 16. J Am Chem Soc. 2008. PMID: 18412341 Free PMC article.
References
-
- Marcus R. A., Sutin N. Electron transfers in chemistry and biology. Biochim. Biophys. Acta. 1985;811:265–322.
-
- Sikorski R. S., Wang L., Markham K. A., Rajagopalan P. T. R., Benkovic S. J., Kohen A. Tunneling and coupled motion in the Escherichia coli dihydrofolate reductase catalysis. J. Am. Chem. Soc. 2004;126:4778–4779. - PubMed
-
- Basran J., Patel S., Sutcliffe M. J., Scrutton N. S. Importance of barrier shape in enzyme-catalyzed reactions: vibrationally assisted hydrogen tunneling in tryptophan tryptophylquinone-dependent amine dehydrogenases. J. Biol. Chem. 2001;276:6234-6242. - PubMed
-
- Francisco W. A., Knapp M. J., Blackburn N. J., Klinman J. P. Hydrogen tunneling in peptidylglycine α-hydroxylating monooxygenase. J. Am. Chem. Soc. 2002;124:8194–8195. - PubMed
-
- Knapp M. J., Klinman J. P. Environmentally coupled hydrogen tunneling: linking catalysis to dynamics. Eur. J. Biochem. 2002;269:3113–3121. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

