Early relief of osteoarthritis symptoms with a natural mineral supplement and a herbomineral combination: a randomized controlled trial [ISRCTN38432711]
- PMID: 16242032
- PMCID: PMC1276811
- DOI: 10.1186/1476-9255-2-11
Early relief of osteoarthritis symptoms with a natural mineral supplement and a herbomineral combination: a randomized controlled trial [ISRCTN38432711]
Abstract
Background: This study was designed to determine if a natural mineral supplement, sierrasil, alone and in combination with a cat's claw extract (Uncaria guianensis), vincaria, has therapeutic potential in mild to moderate osteoarthritis of the knee.
Methods: Patients (n = 107) with mild to moderate osteoarthritis of the knee were randomly assigned to one of 4 groups; high dose sierrasil (3 g/day), low dose sierrasil (2 g/day), low dose sierrasil (2 g/day) + cat's claw extract (100 mg/day) or placebo, administered for 8 weeks. Treatment was double blinded. Primary efficacy variables were WOMAC scores (A, B, C and total). Visual analog score (VAS) for pain, consumption of rescue medication (paracetamol), and tolerability were secondary variables. Safety measures included vital signs and laboratory-based assays.
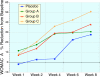
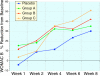
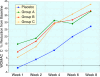
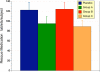
Results: Ninety-one of the 107 patients successfully completed the protocol. All four groups showed improvement in WOMAC and VAS scores after 8 weeks (p < 0.001), in all 3 groups receiving sierrasil the magnitude of benefits were greater vs. placebo (WOMAC Total 38-43% vs. 27%) but this was not statistically significant. In reference to baseline values sierrasil treated groups had a considerably faster onset of benefits. Placebo-treated individuals failed to show significant benefits at 4 weeks (11% reduction in total WOMAC). In contrast, after 1 or 2 weeks of therapy all the sierrasil groups displayed significant reductions in WOMAC scores (p < 0.05) and at week 4 displayed a 38-43% improvement. VAS was significantly improved at 4 weeks in all groups (p < 0.001) but was significantly greater in all sierrasil groups compared to placebo (p < 0.05). Rescue medication use was 28-23% lower in the herbomineral combination and high dose sierrasil groups although not statistically different from placebo (P = 0.101 and P = 0.193, respectively). Tolerability was good for all groups, no serious adverse events were noted and safety parameters remained unchanged.
Conclusion: The natural mineral supplement, sierrasil alone and in combination with a cat's claw extract, improved joint health and function within 1-2 weeks of treatment but significant benefits over placebo were not sustained, possibly due to rescue medication masking. Sierrasil may offer an alternative therapy in subjects with joint pain and dysfunction.
Figures


References
-
- Murray CJL, Lopez AD. The Global Burden of Disease. Boston: Harvard University Press; 1996.
-
- Graham DJ, Campen D, Hui R, Spence M, Cheetham C, Levy G, Shoor S, Ray WA. Risk of acute myocardial infarction and sudden cardiac death in patients treated with cyclo-oxygenase 2 selective and non-selective non-steroidal anti-inflammatory drugs: nested case-control study. Lancet. 2005;365:475–81. - PubMed
-
- Kimmel SE, Berlin JA, Reilly M, Jaskowiak J, Kishel L, Chittams J, Strom BL. Patients exposed to rofecoxib and celecoxib have different odds of nonfatal myocardial infarction. Ann Intern Med. 2005;142:157–64. - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

