A role for airway remodeling during respiratory syncytial virus infection
- PMID: 16242038
- PMCID: PMC1283984
- DOI: 10.1186/1465-9921-6-122
A role for airway remodeling during respiratory syncytial virus infection
Abstract
Background: Severe respiratory syncytial virus infection (RSV) during infancy has been shown to be a major risk factor for the development of subsequent wheeze. However, the reasons for this link remain unclear. The objective of this research was to determine the consequences of early exposure to RSV and allergen in the development of subsequent airway hyperreactivity (AHR) using a developmental time point in the mouse that parallels that of the human neonate.
Methods: Weanling mice were sensitized and challenged with ovalbumin (Ova) and/or infected with RSV. Eight days after the last allergen challenge, various pathophysiological endpoints were examined.
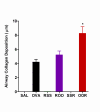
Results: AHR in response to methacholine was enhanced only in weanling mice exposed to Ova and subsequently infected with RSV. The increase in AHR appeared to be unrelated to pulmonary RSV titer. Total bronchoalveolar lavage cellularity in these mice increased approximately two-fold relative to Ova alone and was attributable to increases in eosinophil and lymphocyte numbers. Enhanced pulmonary pathologies including persistent mucus production and subepithelial fibrosis were observed. Interestingly, these data correlated with transient increases in TNF-alpha, IFN-gamma, IL-5, and IL-2.
Conclusion: The observed changes in pulmonary structure may provide an explanation for epidemiological data suggesting that early exposure to allergens and RSV have long-term physiological consequences. Furthermore, the data presented here highlight the importance of preventative strategies against RSV infection of atopic individuals during neonatal development.
Figures





References
-
- Fraenkel DJ, Bardin PG, Sanderson G, Lampe F, Johnston SL, Holgate ST. Lower airways inflammation during rhinovirus colds in normal and in asthmatic subjects. Am J Respir Crit Care Med. 1995;151(3 Pt 1):879–886. - PubMed
-
- Grunberg K, Smits HH, Timmers MC, de Klerk EP, Dolhain RJ, Dick EC, Hiemstra PS, Sterk PJ. Experimental rhinovirus 16 infection. Effects on cell differentials and soluble markers in sputum in asthmatic subjects. Am J Respir Crit Care Med. 1997;156(2 Pt 1):609–616. - PubMed
-
- Glezen WP, Taber LH, Frank AL, Kasel JA. Risk of primary infection and reinfection with respiratory syncytial virus. Am J Dis Child. 1986;140(6):543–546. - PubMed
-
- McConnochie KM, Roghmann KJ. Bronchiolitis as a possible cause of wheezing in childhood: new evidence. Pediatrics. 1984;74(1):1–10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

