Cationic fullerenes are effective and selective antimicrobial photosensitizers
- PMID: 16242655
- PMCID: PMC3071678
- DOI: 10.1016/j.chembiol.2005.08.014
Cationic fullerenes are effective and selective antimicrobial photosensitizers
Abstract
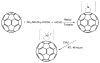
Fullerenes are soccer ball-shaped molecules composed of carbon atoms, and, when derivatized with functional groups, they become soluble and can act as photosensitizers. Antimicrobial photodynamic therapy combines a nontoxic photosensitizer with harmless visible light to generate reactive oxygen species that kill microbial cells. We have compared the antimicrobial activity of six functionalized C(60) compounds with one, two, or three hydrophilic or cationic groups in combination with white light against gram-positive bacteria, gram-negative bacteria, and fungi. After a 10 min incubation, the bis- and tris-cationic fullerenes were highly active in killing all tested microbes (4-6 logs) under conditions in which mammalian cells were comparatively unharmed. These compounds performed significantly better than a widely used antimicrobial photosensitizer, toluidine blue O. The high selectivity and efficacy exhibited by these photosensitizers encourage further testing for antimicrobial applications.
Figures
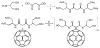





References
-
- Kroto HW, Heath JR, O’Brien SC, Curl RF, Smalley RE. C60: Buckminsterfullerene. Nature. 1985;318:162–163.
-
- Jensen AW, Wilson SR, Schuster DI. Biological applications of fullerenes. Bioorg Med Chem. 1996;4:767–779. - PubMed
-
- Bosi S, Da Ros T, Spalluto G, Prato M. Fullerene derivatives: an attractive tool for biological applications. Eur J Med Chem. 2003;38:913–923. - PubMed
-
- Dugan LL, Lovett EG, Quick KL, Lotharius J, Lin TT, O’Malley KL. Fullerene-based antioxidants and neurodegenerative disorders. Parkinsonism Relat Disord. 2001;7:243–246. - PubMed
-
- Tagmatarchis N, Shinohara H. Fullerenes in medicinal chemistry and their biological applications. Mini Rev Med Chem. 2001;1:339–348. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

