PsbP protein, but not PsbQ protein, is essential for the regulation and stabilization of photosystem II in higher plants
- PMID: 16244145
- PMCID: PMC1283756
- DOI: 10.1104/pp.105.068643
PsbP protein, but not PsbQ protein, is essential for the regulation and stabilization of photosystem II in higher plants
Abstract
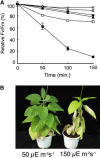
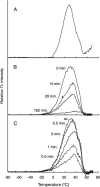
PsbP and PsbQ proteins are extrinsic subunits of photosystem II (PSII) and participate in the normal function of photosynthetic water oxidation. Both proteins exist in a broad range of the oxygenic photosynthetic organisms; however, their physiological roles in vivo have not been well defined in higher plants. In this study, we established and analyzed transgenic tobacco (Nicotiana tabacum) plants in which the levels of PsbP or PsbQ were severely down-regulated by the RNA interference technique. A plant that lacked PsbQ showed no specific phenotype compared to a wild-type plant. This suggests that PsbQ in higher plants is dispensable under the normal growth condition. On the other hand, a plant that lacked PsbP showed prominent phenotypes: drastic retardation of growth, pale-green-colored leaves, and a marked decrease in the quantum yield of PSII evaluated by chlorophyll fluorescence. In PsbP-deficient plant, most PSII core subunits were accumulated in thylakoids, whereas PsbQ, which requires PsbP to bind PSII in vitro, was dramatically decreased. PSII without PsbP was hypersensitive to light and rapidly inactivated when the repair process of the damaged PSII was inhibited by chloramphenicol. Furthermore, thermoluminescence studies showed that the catalytic manganese cluster in PsbP-deficient leaves was markedly unstable and readily disassembled in the dark. The present results demonstrated that PsbP, but not PsbQ, is indispensable for the normal PSII function in higher plants in vivo.
Figures




References
-
- Akabori K, Imaoka A, Toyoshima Y (1984) The role of lipids and 17-kDa protein in enhancing the recovery of O2 evolution in cholate-treated thylakoid membranes. FEBS Lett 173: 36–40
-
- Arató A, Bondarava N, Krieger-Liszkay A (2004) Production of reactive oxygen species in chloride- and calcium-depleted photosystem II and their involvement in photoinhibition. Biochim Biophys Acta 1608: 171–180 - PubMed
-
- Barber J (2004) Water, water everywhere, and its remarkable chemistry. Biochim Biophys Acta 1655: 123–132 - PubMed
-
- Bondarava N, Beyer P, Krieger-Liszkay A (2005) Function of the 23 kDa extrinsic protein of Photosystem II as a manganese binding protein and its role in photoactivation. Biochim Biophys Acta 1708: 63–70 - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources

