Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis
- PMID: 16244149
- PMCID: PMC1283766
- DOI: 10.1104/pp.105.067686
Ascorbic acid deficiency activates cell death and disease resistance responses in Arabidopsis
Abstract
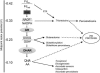
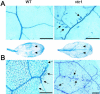
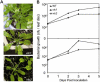
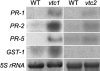
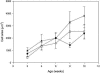
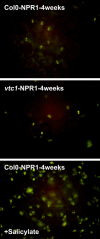
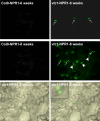
Programmed cell death, developmental senescence, and responses to pathogens are linked through complex genetic controls that are influenced by redox regulation. Here we show that the Arabidopsis (Arabidopsis thaliana) low vitamin C mutants, vtc1 and vtc2, which have between 10% and 25% of wild-type ascorbic acid, exhibit microlesions, express pathogenesis-related (PR) proteins, and have enhanced basal resistance against infections caused by Pseudomonas syringae. The mutants have a delayed senescence phenotype with smaller leaf cells than the wild type at maturity. The vtc leaves have more glutathione than the wild type, with higher ratios of reduced glutathione to glutathione disulfide. Expression of green fluorescence protein (GFP) fused to the nonexpressor of PR protein 1 (GFP-NPR1) was used to detect the presence of NPR1 in the nuclei of transformed plants. Fluorescence was observed in the nuclei of 6- to 8-week-old GFP-NPR1 vtc1 plants, but not in the nuclei of transformed GFP-NPR1 wild-type plants at any developmental stage. The absence of senescence-associated gene 12 (SAG12) mRNA at the time when constitutive cell death and basal resistance were detected confirms that elaboration of innate immune responses in vtc plants does not result from activation of early senescence. Moreover, H2O2-sensitive genes are not induced at the time of systemic acquired resistance execution. These results demonstrate that ascorbic acid abundance modifies the threshold for activation of plant innate defense responses via redox mechanisms that are independent of the natural senescence program.
Figures







Similar articles
-
Ascorbic acid deficiency in arabidopsis induces constitutive priming that is dependent on hydrogen peroxide, salicylic acid, and the NPR1 gene.Mol Plant Microbe Interact. 2010 Mar;23(3):340-51. doi: 10.1094/MPMI-23-3-0340. Mol Plant Microbe Interact. 2010. PMID: 20121455
-
The secondary metabolism glycosyltransferases UGT73B3 and UGT73B5 are components of redox status in resistance of Arabidopsis to Pseudomonas syringae pv. tomato.Plant Cell Environ. 2014 May;37(5):1114-29. doi: 10.1111/pce.12221. Epub 2013 Nov 24. Plant Cell Environ. 2014. PMID: 24131360
-
Low antioxidant concentrations impact on multiple signalling pathways in Arabidopsis thaliana partly through NPR1.J Exp Bot. 2012 Mar;63(5):1849-61. doi: 10.1093/jxb/err358. Epub 2012 Jan 2. J Exp Bot. 2012. PMID: 22213815 Free PMC article.
-
The effects of ozone on antioxidant responses in plants.Free Radic Biol Med. 1997;23(3):480-8. doi: 10.1016/s0891-5849(97)00108-1. Free Radic Biol Med. 1997. PMID: 9214586 Review.
-
Interactions between biosynthesis, compartmentation and transport in the control of glutathione homeostasis and signalling.J Exp Bot. 2002 May;53(372):1283-304. doi: 10.1093/jexbot/53.372.1283. J Exp Bot. 2002. PMID: 11997376 Review.
Cited by
-
Reduced carbohydrate availability enhances the susceptibility of Arabidopsis toward Colletotrichum higginsianum.Plant Physiol. 2013 May;162(1):225-38. doi: 10.1104/pp.112.209676. Epub 2013 Mar 13. Plant Physiol. 2013. PMID: 23487433 Free PMC article.
-
Microscopic and Transcriptomic Comparison of Powdery Mildew Resistance in the Progenies of Brassica carinata × B. napus.Int J Mol Sci. 2022 Sep 1;23(17):9961. doi: 10.3390/ijms23179961. Int J Mol Sci. 2022. PMID: 36077359 Free PMC article.
-
Wheat yellow mosaic virus NIb targets TaVTC2 to elicit broad-spectrum pathogen resistance in wheat.Plant Biotechnol J. 2023 May;21(5):1073-1088. doi: 10.1111/pbi.14019. Epub 2023 Feb 14. Plant Biotechnol J. 2023. PMID: 36715229 Free PMC article.
-
Comparative changes in the antioxidant system in the flag leaf of early and normally senescing near-isogenic lines of wheat (Triticum aestivum L.).Plant Cell Rep. 2014 Jul;33(7):1109-20. doi: 10.1007/s00299-014-1600-0. Epub 2014 Apr 1. Plant Cell Rep. 2014. PMID: 24687459
-
Jasmonate Regulates Plant Responses to Postsubmergence Reoxygenation through Transcriptional Activation of Antioxidant Synthesis.Plant Physiol. 2017 Mar;173(3):1864-1880. doi: 10.1104/pp.16.01803. Epub 2017 Jan 12. Plant Physiol. 2017. PMID: 28082717 Free PMC article.
References
-
- Alvarez ME, Pennell RI, Meijer PJ, Ishikawa A, Dixon RA, Lamb C (1998) Reactive oxygen intermediates mediate a systemic signal network in the establishment of plant immunity. Cell 20: 773–784 - PubMed
-
- Apel K, Hirt H (2004) Reactive oxygen species: metabolism, oxidative stress, and signal transduction. Annu Rev Plant Physiol Plant Mol Biol 55: 373–399 - PubMed
-
- Arrigoni O, de Tullio MC (2000) The role of ascorbic acid in cell metabolism: between gene-directed functions and unpredictable chemical reactions. J Plant Physiol 157: 481–488
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

