Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles
- PMID: 16244709
- PMCID: PMC1262624
- DOI: 10.1371/journal.ppat.0010017
Identification of the moving junction complex of Toxoplasma gondii: a collaboration between distinct secretory organelles
Abstract
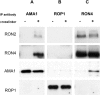
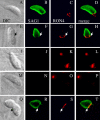
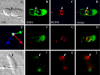
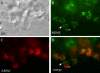
Apicomplexan parasites, including Toxoplasma gondii and Plasmodium sp., are obligate intracellular protozoa. They enter into a host cell by attaching to and then creating an invagination in the host cell plasma membrane. Contact between parasite and host plasma membranes occurs in the form of a ring-shaped moving junction that begins at the anterior end of the parasite and then migrates posteriorly. The resulting invagination of host plasma membrane creates a parasitophorous vacuole that completely envelops the now intracellular parasite. At the start of this process, apical membrane antigen 1 (AMA1) is released onto the parasite surface from specialized secretory organelles called micronemes. The T. gondii version of this protein, TgAMA1, has been shown to be essential for invasion but its exact role has not previously been determined. We identify here a trio of proteins that associate with TgAMA1, at least one of which associates with TgAMA1 at the moving junction. Surprisingly, these new proteins derive not from micronemes, but from the anterior secretory organelles known as rhoptries and specifically, for at least two, from the neck portion of these club-shaped structures. Homologues for these AMA1-associated proteins are found throughout the Apicomplexa strongly suggesting that this moving junction apparatus is a conserved feature of this important class of parasites. Differences between the contributing proteins in different species may, in part, be the result of selective pressure from the different niches occupied by these parasites.
Conflict of interest statement
Figures


References
-
- Carruthers VB, Sibley LD. Sequential protein secretion from three distinct organelles of Toxoplasma gondii accompanies invasion of human fibroblasts. Eur J Cell Biol. 1997;73:114–123. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
- R01 AI021423/AI/NIAID NIH HHS/United States
- F32 AI010552/AI/NIAID NIH HHS/United States
- R37 AI021423/AI/NIAID NIH HHS/United States
- AI45057/AI/NIAID NIH HHS/United States
- Z01 AI005093/ImNIH/Intramural NIH HHS/United States
- R01 AI045806/AI/NIAID NIH HHS/United States
- R01 AI063276/AI/NIAID NIH HHS/United States
- AI21423/AI/NIAID NIH HHS/United States
- F32AI10552/AI/NIAID NIH HHS/United States
- WT_/Wellcome Trust/United Kingdom
- 1R01AI045806-01A1/AI/NIAID NIH HHS/United States
- AI063276/AI/NIAID NIH HHS/United States
- AI05093/AI/NIAID NIH HHS/United States
- R01 AI045057/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources

