Effect of a clinical trial alert system on physician participation in trial recruitment
- PMID: 16246994
- PMCID: PMC1343501
- DOI: 10.1001/archinte.165.19.2272
Effect of a clinical trial alert system on physician participation in trial recruitment
Abstract
Background: Failure to recruit a sufficient number of eligible subjects in a timely manner represents a major impediment to the success of clinical trials. Physician participation is vital to trial recruitment but is often limited.
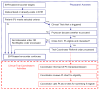
Methods: After 12 months of traditional recruitment to a clinical trial, we activated our electronic health record (EHR)-based clinical trial alert (CTA) system in selected outpatient clinics of a large, US academic health care system. When a patient's EHR data met selected trial criteria during the subsequent 4-month intervention period, the CTA prompted physician consideration of the patient's eligibility and facilitated secure messaging to the trial's coordinator. Subjects were the 114 physicians practicing at selected EHR-equipped clinics throughout our study. We compared differences in the number of physicians participating in recruitment and their recruitment rates before and after CTA activation.
Results: The CTA intervention was associated with significant increases in the number of physicians generating referrals (5 before and 42 after; P < .001) and enrollments (5 before and 11 after; P = .03), a 10-fold increase in those physicians' referral rate (5.7/mo before and 59.5/mo after; rate ratio, 10.44; 95% confidence interval, 7.98-13.68; P<.001), and a doubling of their enrollment rate (2.9/mo before and 6.0/mo after; rate ratio, 2.06; 95% confidence interval, 1.22-3.46; P = .007).
Conclusions: Use of an EHR-based CTA led to significant increases in physicians' participation in and recruitment rates to an ongoing clinical trial. Given the trend toward the EHR implementation in health care centers engaged in clinical research, this approach may represent a much-needed solution to the common problem of inadequate trial recruitment.
Figures
Comment in
-
In search of evidence: is there the will and a way?Arch Intern Med. 2005 Oct 24;165(19):2194-5. doi: 10.1001/archinte.165.19.2194. Arch Intern Med. 2005. PMID: 16246979 No abstract available.
References
-
- Nathan DG, Wilson JD. Clinical Research and the NIH -- A Report Card. N Engl J Med. November 6 2003 2003;349(19):1860–1865. - PubMed
-
- Campbell EG, Weissman JS, Moy E, Blumenthal D. Status of clinical research in academic health centers: views from the research leadership. JAMA. Aug 15 2001;286(7):800–806. - PubMed
-
- Hunninghake DB, Darby CA, Probstfield JL. Recruitment experience in clinical trials: literature summary and annotated bibliography. Control Clin Trials. Dec 1987;8(4 Suppl):6S–30S. - PubMed
-
- Marks L, Power E. Using technology to address recruitment issues in the clinical trial process. Trends Biotechnol. Mar 2002;20(3):105–109. - PubMed
-
- Sung NS, Crowley WF, Jr, Genel M, et al. Central challenges facing the national clinical research enterprise. JAMA. Mar 12 2003;289(10):1278–1287. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous