Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations
- PMID: 16247029
- PMCID: PMC2171199
- DOI: 10.1083/jcb.200507054
Termination of cAMP signals by Ca2+ and G(alpha)i via extracellular Ca2+ sensors: a link to intracellular Ca2+ oscillations
Abstract
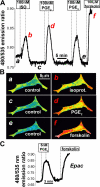
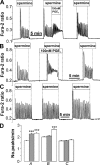
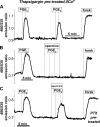
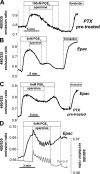
Termination of cyclic adenosine monophosphate (cAMP) signaling via the extracellular Ca(2+)-sensing receptor (CaR) was visualized in single CaR-expressing human embryonic kidney (HEK) 293 cells using ratiometric fluorescence resonance energy transfer-dependent cAMP sensors based on protein kinase A and Epac. Stimulation of CaR rapidly reversed or prevented agonist-stimulated elevation of cAMP through a dual mechanism involving pertussis toxin-sensitive Galpha(i) and the CaR-stimulated increase in intracellular [Ca2+]. In parallel measurements with fura-2, CaR activation elicited robust Ca2+ oscillations that increased in frequency in the presence of cAMP, eventually fusing into a sustained plateau. Considering the Ca2+ sensitivity of cAMP accumulation in these cells, lack of oscillations in [cAMP] during the initial phases of CaR stimulation was puzzling. Additional experiments showed that low-frequency, long-duration Ca2+ oscillations generated a dynamic staircase pattern in [cAMP], whereas higher frequency spiking had no effect. Our data suggest that the cAMP machinery in HEK cells acts as a low-pass filter disregarding the relatively rapid Ca2+ spiking stimulated by Ca(2+)-mobilizing agonists under physiological conditions.
Figures


Similar articles
-
Phosphatidylinositol 3-kinase offsets cAMP-mediated positive inotropic effect via inhibiting Ca2+ influx in cardiomyocytes.Circ Res. 2004 Dec 10;95(12):1183-90. doi: 10.1161/01.RES.0000150049.74539.8a. Epub 2004 Nov 11. Circ Res. 2004. PMID: 15539636
-
CaR activation increases TNF production by mTAL cells via a Gi-dependent mechanism.Am J Physiol Renal Physiol. 2008 Feb;294(2):F345-54. doi: 10.1152/ajprenal.00509.2006. Epub 2007 Nov 21. Am J Physiol Renal Physiol. 2008. PMID: 18032544
-
Ca2+ stimulation of adenylyl cyclase generates dynamic oscillations in cyclic AMP.J Cell Sci. 2006 Mar 1;119(Pt 5):828-36. doi: 10.1242/jcs.02812. Epub 2006 Feb 14. J Cell Sci. 2006. PMID: 16478784
-
Review article: cyclic AMP sensors in living cells: what signals can they actually measure?Ann Biomed Eng. 2002 Sep;30(8):1088-99. doi: 10.1114/1.1511242. Ann Biomed Eng. 2002. PMID: 12449769 Review.
-
Calcium signaling in vasopressin-induced aquaporin-2 trafficking.Pflugers Arch. 2008 Jul;456(4):747-54. doi: 10.1007/s00424-007-0371-7. Epub 2007 Oct 24. Pflugers Arch. 2008. PMID: 17957381 Review.
Cited by
-
Calcium-Sensing Receptor and Regulation of WNK Kinases in the Kidney.Cells. 2020 Jul 9;9(7):1644. doi: 10.3390/cells9071644. Cells. 2020. PMID: 32659887 Free PMC article. Review.
-
Effect of calcium-sensing receptor activation in models of autosomal recessive or dominant polycystic kidney disease.Nephrol Dial Transplant. 2009 Feb;24(2):526-34. doi: 10.1093/ndt/gfn527. Epub 2008 Sep 30. Nephrol Dial Transplant. 2009. PMID: 18826972 Free PMC article.
-
Oscillating cortical thick ascending limb cells at the juxtaglomerular apparatus.J Am Soc Nephrol. 2008 Oct;19(10):1940-6. doi: 10.1681/ASN.2007080900. Epub 2008 Jun 18. J Am Soc Nephrol. 2008. PMID: 18562570 Free PMC article.
-
"cAMP sponge": a buffer for cyclic adenosine 3', 5'-monophosphate.PLoS One. 2009 Nov 3;4(11):e7649. doi: 10.1371/journal.pone.0007649. PLoS One. 2009. PMID: 19888343 Free PMC article.
-
Pseudomonas aeruginosa Homoserine lactone activates store-operated cAMP and cystic fibrosis transmembrane regulator-dependent Cl- secretion by human airway epithelia.J Biol Chem. 2010 Nov 5;285(45):34850-63. doi: 10.1074/jbc.M110.167668. Epub 2010 Aug 25. J Biol Chem. 2010. PMID: 20739289 Free PMC article.
References
-
- Adams, S.R., A.T. Harootunian, Y.J. Buechler, S.S. Taylor, and R.Y. Tsien. 1991. Fluorescence ratio imaging of cyclic AMP in single cells. Nature. 349:694–697. - PubMed
-
- Beavo, J.A., and L.L. Brunton. 2002. Cyclic nucleotide research—still expanding after half a century. Nat. Rev. Mol. Cell Biol. 3:710–718. - PubMed
-
- Berridge, M.J., P. Lipp, and M.D. Bootman. 2000. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 1:11–21. - PubMed
-
- Breitwieser, G.E., and L. Gama. 2001. Calcium-sensing receptor activation induces intracellular calcium oscillations. Am. J. Physiol. Cell Physiol. 280:C1412–C1421. - PubMed
-
- Breitwieser, G.E., S.U. Miedlich, and M. Zhang. 2004. Calcium sensing receptors as integrators of multiple metabolic signals. Cell Calcium. 35:209–216. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

