Pyroglutamyl peptidase type I from Trypanosoma brucei: a new virulence factor from African trypanosomes that de-blocks regulatory peptides in the plasma of infected hosts
- PMID: 16248854
- PMCID: PMC1383713
- DOI: 10.1042/BJ20051593
Pyroglutamyl peptidase type I from Trypanosoma brucei: a new virulence factor from African trypanosomes that de-blocks regulatory peptides in the plasma of infected hosts
Abstract
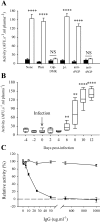
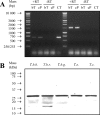
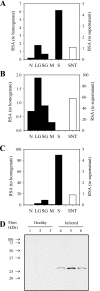
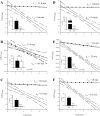
Peptidases of parasitic protozoans are emerging as novel virulence factors and therapeutic targets in parasitic infections. A trypanosome-derived aminopeptidase that exclusively hydrolysed substrates with Glp (pyroglutamic acid) in P1 was purified 9248-fold from the plasma of rats infected with Trypanosoma brucei brucei. The enzyme responsible was cloned from a T. brucei brucei genomic DNA library and identified as type I PGP (pyroglutamyl peptidase), belonging to the C15 family of cysteine peptidases. We showed that PGP is expressed in all life cycle stages of T. brucei brucei and is expressed in four other blood-stream-form African trypanosomes. Trypanosome PGP was optimally active and stable at bloodstream pH, and was insensitive to host plasma cysteine peptidase inhibitors. Native purified and recombinant hyper-expressed trypanosome PGP removed the N-terminal Glp blocking groups from TRH (thyrotrophin-releasing hormone) and GnRH (gonadotropin-releasing hormone) with a k(cat)/K(m) value of 0.5 and 0.1 s(-1) x microM(-1) respectively. The half-life of TRH and GnRH was dramatically reduced in the plasma of trypanosome-infected rats, both in vitro and in vivo. Employing an activity-neutralizing anti-trypanosome PGP antibody, and pyroglutamyl diazomethyl ketone, a specific inhibitor of type I PGP, we demonstrated that trypanosome PGP is entirely responsible for the reduced plasma half-life of TRH, and partially responsible for the reduced plasma half-life of GnRH in a rodent model of African trypanosomiasis. The abnormal degradation of TRH and GnRH, and perhaps other neuropeptides N-terminally blocked with a pyroglutamyl moiety, by trypanosome PGP, may contribute to some of the endocrine lesions observed in African trypanosomiasis.
Figures


Similar articles
-
Trypanosome-derived oligopeptidase B is released into the plasma of infected rodents, where it persists and retains full catalytic activity.Infect Immun. 2001 Apr;69(4):2757-61. doi: 10.1128/IAI.69.4.2757-2761.2001. Infect Immun. 2001. PMID: 11254649 Free PMC article.
-
Evidence for pyroglutamyl peptidase I and prolyl endopeptidase activities in the rat insulinoma cell line RINm 5F: lack of relationship with TRH metabolism.Mol Cell Biochem. 1991 Jul 24;106(1):15-24. doi: 10.1007/BF00231184. Mol Cell Biochem. 1991. PMID: 1681421
-
Tropolysin, a new oligopeptidase from African trypanosomes.Biochemistry. 2005 Nov 8;44(44):14658-69. doi: 10.1021/bi051035k. Biochemistry. 2005. PMID: 16262265
-
Pyroglutamyl peptidase: an overview of the three known enzymatic forms.Biochim Biophys Acta. 1998 Dec 8;1429(1):1-17. doi: 10.1016/s0167-4838(98)00248-9. Biochim Biophys Acta. 1998. PMID: 9920379 Review.
-
The chemistry and biology of trypanosomal trans-sialidases: virulence factors in Chagas disease and sleeping sickness.Chembiochem. 2011 Oct 17;12(15):2246-64. doi: 10.1002/cbic.201100421. Epub 2011 Sep 28. Chembiochem. 2011. PMID: 21956798 Review.
Cited by
-
Parasite prolyl oligopeptidases and the challenge of designing chemotherapeuticals for Chagas disease, leishmaniasis and African trypanosomiasis.Curr Med Chem. 2013;20(25):3103-15. doi: 10.2174/0929867311320250006. Curr Med Chem. 2013. PMID: 23514419 Free PMC article. Review.
-
The Structural Basis of Peptide Binding at Class A G Protein-Coupled Receptors.Molecules. 2021 Dec 30;27(1):210. doi: 10.3390/molecules27010210. Molecules. 2021. PMID: 35011444 Free PMC article. Review.
-
Comparative genomics of canine-isolated Leishmania (Leishmania) amazonensis from an endemic focus of visceral leishmaniasis in Governador Valadares, southeastern Brazil.Sci Rep. 2017 Jan 16;7:40804. doi: 10.1038/srep40804. Sci Rep. 2017. PMID: 28091623 Free PMC article.
-
Characterization of the M32 metallocarboxypeptidase of Trypanosoma brucei: differences and similarities with its orthologue in Trypanosoma cruzi.Mol Biochem Parasitol. 2012 Aug;184(2):63-70. doi: 10.1016/j.molbiopara.2012.04.008. Epub 2012 Apr 28. Mol Biochem Parasitol. 2012. PMID: 22575602 Free PMC article.
-
The isoenzyme of glutaminyl cyclase is an important regulator of monocyte infiltration under inflammatory conditions.EMBO Mol Med. 2011 Sep;3(9):545-58. doi: 10.1002/emmm.201100158. Epub 2011 Jul 20. EMBO Mol Med. 2011. PMID: 21774078 Free PMC article.
References
-
- Barrett M. P., Burchmore R. J., Stich A., Lazzari J. O., Frasch A. C., Cazzulo J. J., Krishna S. The trypanosomiases. Lancet. 2003;362:1469–1480. - PubMed
-
- Boutignon F., Huet-Duvillier G., Demeyer D., Richet C., Degand P. Study of proteolytic activities released by incubation of trypanosomes (Trypanosoma brucei brucei) in pH 5.5 and pH 7.0 phosphate/glucose buffers. Biochim. Biophys. Acta. 1990;1035:369–377. - PubMed
-
- Knowles G., Black S. J., Whitelaw D. D. Peptidase in the plasma of mice infected with Trypanosoma brucei brucei. Parasitology. 1987;95:291–300. - PubMed
-
- Tetaert D., Soudan B., Huet-Duvillier G., Degand P., Boersma A. Unusual cleavage of peptidic hormones generated by trypanosome enzymes released in infested rat serum. Int. J. Pept. Protein Res. 1993;41:147–152. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
LinkOut - more resources
Full Text Sources
Miscellaneous

