Recruitment of governing elements for electron transfer in the nitric oxide synthase family
- PMID: 16249336
- PMCID: PMC1276075
- DOI: 10.1073/pnas.0506522102
Recruitment of governing elements for electron transfer in the nitric oxide synthase family
Abstract
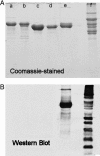
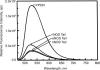
At least three building blocks are responsible for the molecular basis of the modulation of electron transfer in nitric oxide synthase (NOS) isoforms: the calmodulin-binding sequence, the C-terminal extension, and the autoregulatory loop in the reductase domain. We have attempted to impart the control conferred by the C termini of NOS to cytochrome P450 oxidoreductase (CYPOR), which contains none of these regulatory elements. The effect of these C termini on the properties of CYPOR sheds light on the possible evolutionary origin of NOS and addresses the recruitment of new peptides on the development of new functions for CYPOR. The C termini of NOSs modulate flavoprotein-mediated electron transfer to various electron acceptors. The reduction of the artificial electron acceptors cytochrome c, 2,6-dichlorophenolindophenol, and ferricyanide was inhibited by the addition of any of these C termini to CYPOR, whereas the reduction of molecular O(2) was increased. This suggests a shift in the rate-limiting step, indicating that the NOS C termini interrupt electron flux between flavin mononucleotide (FMN) and flavin adenine dinucleotide (FAD) and/or the electron acceptors. The modulation of CYPOR by the addition of the NOS C termini is also supported by flavin reoxidation and fluorescence-quenching studies and antibody recognition of the C-terminal extension. These experiments support the origin of the NOS enzymes from modules consisting of a heme domain and CYPOR or ferredoxin-NADP(+) reductase- and flavodoxin-like subdomains that constitute CYPOR, followed by further recruitment of smaller modulating elements into the flavin-binding domains.
Figures
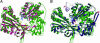
References
-
- Lewis, D. F. V. (2001) Guide to Cytochromes P450: Structure and Function (Taylor and Francis, New York).
-
- Massey, V. (2000) Biochem. Soc. Trans. 28, 283-296. - PubMed
-
- Masters, B. S. S., Baron, J., Taylor, W. E., Isaacson, E. L. & LoSpalluto, J. (1971) J. Biol. Chem. 246, 4143-4150. - PubMed
-
- Schacter, B. A., Nelson, E. B., Marver, H. S. & Masters, B. S. S. (1972) J. Biol. Chem. 247, 3601-3607. - PubMed
-
- Ono, T. & Bloch, K. (1975) J. Biol. Chem. 250, 1571-1579. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

