Nitric oxide and cGMP protein kinase (cGK) regulate dendritic-cell migration toward the lymph-node-directing chemokine CCL19
- PMID: 16249377
- PMCID: PMC1895400
- DOI: 10.1182/blood-2005-07-2901
Nitric oxide and cGMP protein kinase (cGK) regulate dendritic-cell migration toward the lymph-node-directing chemokine CCL19
Abstract
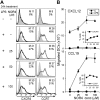
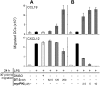
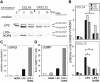
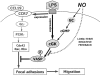
Dendritic-cell (DC) migration to secondary lymphoid organs is crucial for the initiation of adaptive immune responses. Although LPS up-regulates CCR7 on DCs, a second signal is required to enable them to migrate toward the chemokine CCL19 (MIP-3beta). We found that the nitric oxide (NO) donor NOR4 provides a signal allowing LPS-stimulated DCs to migrate toward CCL19. NO affects DC migration through both the initial activation of the cGMP/cGMP kinase (cGMP/cGK) pathway and a long-term effect that reduced cGK activity via negative feedback. Indeed, migration of DCs toward CCL19, unlike migration toward CXCL12 (SDF-1alpha), required inhibition of cGK. LPS increased both cGK expression and cGK activity as measured by phosphorylation of the key cGK target vasodilator-stimulated phosphoprotein (VASP). Because cGK phosphorylation of VASP can disrupt focal adhesions and inhibit cell migration, LPS-induced VASP phosphorylation may prevent DCs from migrating without a second signal. Long-term NOR4 treatment inhibited the increase in cGK-dependent VASP phosphorylation, releasing this brake so that DCs can migrate. NO has been implicated in the regulation of autoimmunity through its effect on T cells. Our results suggest that NO regulation of DC migration and cytokine production may contribute to the protective effects of NO in autoimmune disorders.
Figures
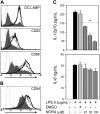
 ) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO (
) or absence (▪) of graded doses of NOR4 (25, 50, 100 μM) or the vehicle control DMSO ( ). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant
). Supernatants were collected and analyzed for IL-12p70 and IL-6 by ELISA. *P < .001 compared with control LPS-treated DCs (▪), unpaired Student t test. Data are from 1 representative experiment of 9 independent experiments performed using cells from different donors. A statistical analysis of the 9 experiments using the paired t test gave a value of P = .01, confirming that the inhibition of IL-12p70 production by NOR4 is statistically significant

Similar articles
-
Cyclic nucleotides promote monocyte differentiation toward a DC-SIGN+ (CD209) intermediate cell and impair differentiation into dendritic cells.J Immunol. 2003 Dec 15;171(12):6421-30. doi: 10.4049/jimmunol.171.12.6421. J Immunol. 2003. PMID: 14662841
-
Regulation of human endothelial cell focal adhesion sites and migration by cGMP-dependent protein kinase I.J Biol Chem. 2000 Aug 18;275(33):25723-32. doi: 10.1074/jbc.M909632199. J Biol Chem. 2000. PMID: 10851246
-
KT5823 inhibits cGMP-dependent protein kinase activity in vitro but not in intact human platelets and rat mesangial cells.J Biol Chem. 2000 Oct 27;275(43):33536-41. doi: 10.1074/jbc.M005670200. J Biol Chem. 2000. PMID: 10922374
-
Functional analysis of cGMP-dependent protein kinases I and II as mediators of NO/cGMP effects.Naunyn Schmiedebergs Arch Pharmacol. 1998 Jul;358(1):134-9. doi: 10.1007/pl00005234. Naunyn Schmiedebergs Arch Pharmacol. 1998. PMID: 9721015 Review.
-
Tracking functions of cGMP-dependent protein kinases (cGK).Front Biosci. 2005 May 1;10:1313-28. doi: 10.2741/1621. Front Biosci. 2005. PMID: 15769627 Review.
Cited by
-
Constitutively Activated DAP12 Induces Functional Anti-Tumor Activation and Maturation of Human Monocyte-Derived DC.Int J Mol Sci. 2021 Jan 27;22(3):1241. doi: 10.3390/ijms22031241. Int J Mol Sci. 2021. PMID: 33513928 Free PMC article.
-
Normoxic cyclic GMP-independent oxidative signaling by nitrite enhances airway epithelial cell proliferation and wound healing.Nitric Oxide. 2012 May 15;26(4):203-10. doi: 10.1016/j.niox.2012.03.002. Epub 2012 Mar 8. Nitric Oxide. 2012. PMID: 22425780 Free PMC article.
-
Ena/VASP proteins at the crossroads of actin nucleation pathways in dendritic cell migration.Front Cell Dev Biol. 2022 Oct 3;10:1008898. doi: 10.3389/fcell.2022.1008898. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 36274843 Free PMC article.
-
Identification of gene expression patterns crucially involved in experimental autoimmune encephalomyelitis and multiple sclerosis.Dis Model Mech. 2016 Oct 1;9(10):1211-1220. doi: 10.1242/dmm.025536. Epub 2016 Aug 12. Dis Model Mech. 2016. PMID: 27519689 Free PMC article.
-
Re-thinking the functions of IgA(+) plasma cells.Gut Microbes. 2014;5(5):652-62. doi: 10.4161/19490976.2014.969977. Gut Microbes. 2014. PMID: 25483334 Free PMC article. Review.
References
-
- Banchereau J, Briere F, Caux C, et al. Immunobiology of dendritic cells. Annu Rev Immunol. 2000; 18: 767-811. - PubMed
-
- Ricciardi-Castagnoli P, Granucci F. Opinion: interpretation of the complexity of innate immune responses by functional genomics. Nat Rev Immunol. 2002;2: 881-889. - PubMed
-
- Sallusto F. Origin and migratory properties of dendritic cells in the skin. Curr Opin Allergy Clin Immunol. 2001;1: 441-448. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

