An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor
- PMID: 16249382
- PMCID: PMC1379661
- DOI: 10.1182/blood-2005-06-2600
An amphipathic motif at the transmembrane-cytoplasmic junction prevents autonomous activation of the thrombopoietin receptor
Abstract
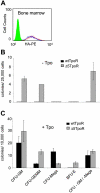
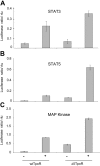
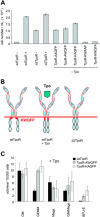
Ligand binding to the thrombopoietin receptor (TpoR) is thought to impose a dimeric receptor conformation(s) leading to hematopoietic stem cell renewal, megakaryocyte differentiation, and platelet formation. Unlike other cytokine receptors, such as the erythropoietin receptor, TpoR contains an amphipathic KWQFP motif at the junction between the transmembrane (TM) and cytoplasmic domains. We show here that a mutant TpoR (delta5TpoR), where this sequence was deleted, is constitutively active. In the absence of ligand, delta5TpoR activates Jak2, Tyk2, STAT5, and mitogen-activated protein (MAP) kinase, but does not appear to induce STAT3 phosphorylation. Delta5TpoR induces hematopoietic myeloid differentiation in the absence of Tpo. In the presence of Tpo, the delta5TpoR mutant appears to enhance erythroid differentiation when compared with the Tpo-activated wild-type TpoR. Strikingly, individual substitution of K507 or W508 to alanine also induces constitutive TpoR activation, indicating that the K and W residues within the amphipathic KWQFP motif are crucial for maintaining the unliganded receptor inactive. These residues may be targets for activating mutations in humans. Such a motif may exist in other receptors to prevent ligand-independent activation and to allow signaling via multiple flexible interfaces.
Figures


Similar articles
-
Janus kinases affect thrombopoietin receptor cell surface localization and stability.J Biol Chem. 2005 Jul 22;280(29):27251-61. doi: 10.1074/jbc.M501376200. Epub 2005 May 16. J Biol Chem. 2005. PMID: 15899890
-
Role of the distal half of the c-Mpl intracellular domain in control of platelet production by thrombopoietin in vivo.Mol Cell Biol. 2000 Jan;20(2):507-15. doi: 10.1128/MCB.20.2.507-515.2000. Mol Cell Biol. 2000. PMID: 10611229 Free PMC article.
-
A novel MPL point mutation resulting in thrombopoietin-independent activation.Leukemia. 2002 Aug;16(8):1500-6. doi: 10.1038/sj.leu.2402554. Leukemia. 2002. PMID: 12145691
-
Thrombopoietin signal transduction: studies from cell lines and primary cells.Methods. 1999 Mar;17(3):238-49. doi: 10.1006/meth.1998.0734. Methods. 1999. PMID: 10080909 Review.
-
Thrombopoietin and its receptor.Eur Cytokine Netw. 1998 Sep;9(3):221-31. Eur Cytokine Netw. 1998. PMID: 9831170 Review.
Cited by
-
Juxtamembrane contribution to transmembrane signaling.Biopolymers. 2015 Jul;104(4):317-22. doi: 10.1002/bip.22651. Biopolymers. 2015. PMID: 25846274 Free PMC article. Review.
-
Thrombopoietin receptor activation: transmembrane helix dimerization, rotation, and allosteric modulation.FASEB J. 2011 Jul;25(7):2234-44. doi: 10.1096/fj.10-178673. Epub 2011 Mar 14. FASEB J. 2011. PMID: 21402716 Free PMC article.
-
Molecular classification of myeloproliferative neoplasms-pros and cons.Curr Hematol Malig Rep. 2013 Dec;8(4):342-50. doi: 10.1007/s11899-013-0179-9. Curr Hematol Malig Rep. 2013. PMID: 24091831 Review.
-
Genetic Alterations of the Thrombopoietin/MPL/JAK2 Axis Impacting Megakaryopoiesis.Front Endocrinol (Lausanne). 2017 Sep 12;8:234. doi: 10.3389/fendo.2017.00234. eCollection 2017. Front Endocrinol (Lausanne). 2017. PMID: 28955303 Free PMC article. Review.
-
Molecular mechanisms associated with leukemic transformation of MPL-mutant myeloproliferative neoplasms.Haematologica. 2010 Dec;95(12):2153-6. doi: 10.3324/haematol.2010.029306. Epub 2010 Sep 7. Haematologica. 2010. PMID: 20823136 Free PMC article.
References
-
- de Sauvage FJ, Hass PE, Spencer SD, et al. Stimulation of megakaryocytopoiesis and thrombopoiesis by the c-Mpl ligand. Nature. 1994;369: 533-538. - PubMed
-
- Kaushansky K, Lok S, Holly RD, et al. Promotion of megakaryocyte progenitor expansion and differentiation by the c-Mpl ligand thrombopoietin. Nature. 1994;369: 568-571. - PubMed
-
- Solar GP, Kerr WG, Zeigler FC, et al. Role of c-mpl in early hematopoiesis. Blood. 1998;92: 4-10. - PubMed
-
- Miyakawa Y, Oda A, Druker BJ, et al. Recombinant thrombopoietin induces rapid protein tyrosine phosphorylation of Janus kinase 2 and Shc in human blood platelets. Blood. 1995;86: 23-27. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

