Olfactory cortical adaptation facilitates detection of odors against background
- PMID: 16251260
- PMCID: PMC2292127
- DOI: 10.1152/jn.00812.2005
Olfactory cortical adaptation facilitates detection of odors against background
Abstract
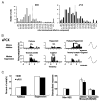
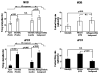
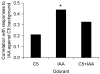
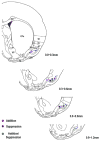
Detection and discrimination of odors generally, if not always, occurs against an odorous background. On any given inhalation, olfactory receptor neurons will be activated by features of both the target odorant and features of background stimuli. To identify a target odorant against a background therefore, the olfactory system must be capable of grouping a subset of features into an odor object distinct from the background. Our previous work has suggested that rapid homosynaptic depression of afferents to the anterior piriform cortex (aPCX) contributes to both cortical odor adaptation to prolonged stimulation and habituation of simple odor-evoked behaviors. We hypothesize here that this process may also contribute to figure-ground separation of a target odorant from background stimulation. Single-unit recordings were made from both mitral/tufted cells and aPCX neurons in urethan-anesthetized rats and mice. Single-unit responses to odorant stimuli and their binary mixtures were determined. One of the odorants was randomly selected as the background and presented for 50 s. Forty seconds after the onset of the background stimulus, the second target odorant was presented, producing a binary mixture. The results suggest that mitral/tufted cells continue to respond to the background odorant and, when the target odorant is presented, had response magnitudes similar to that evoked by the binary mixture. In contrast, aPCX neurons filter out the background stimulus while maintaining responses to the target stimulus. Thus the aPCX acts as a filter driven most strongly by changing stimuli, providing a potential mechanism for olfactory figure-ground separation and selective reading of olfactory bulb output.
Figures


Similar articles
-
Rapid, experience-induced enhancement in odorant discrimination by anterior piriform cortex neurons.J Neurophysiol. 2003 Jul;90(1):65-72. doi: 10.1152/jn.00133.2003. Epub 2003 Mar 26. J Neurophysiol. 2003. PMID: 12660351
-
Comparison of odor receptive field plasticity in the rat olfactory bulb and anterior piriform cortex.J Neurophysiol. 2000 Dec;84(6):3036-42. doi: 10.1152/jn.2000.84.6.3036. J Neurophysiol. 2000. PMID: 11110830
-
Separate encoding of identity and similarity of complex familiar odors in piriform cortex.Proc Natl Acad Sci U S A. 2006 Oct 10;103(41):15206-11. doi: 10.1073/pnas.0604313103. Epub 2006 Sep 27. Proc Natl Acad Sci U S A. 2006. PMID: 17005727 Free PMC article.
-
Receptive fields in the rat piriform cortex.Chem Senses. 2001 Jun;26(5):577-84. doi: 10.1093/chemse/26.5.577. Chem Senses. 2001. PMID: 11418503 Review.
-
Acetylcholine and olfactory perceptual learning.Learn Mem. 2004 Jan-Feb;11(1):28-34. doi: 10.1101/lm.66404. Learn Mem. 2004. PMID: 14747514 Free PMC article. Review.
Cited by
-
The Olfactory Mosaic: Bringing an Olfactory Network Together for Odor Perception.Perception. 2017 Mar-Apr;46(3-4):320-332. doi: 10.1177/0301006616663216. Epub 2016 Sep 29. Perception. 2017. PMID: 27687814 Free PMC article. Review.
-
Neural computations with mammalian infochemicals.J Chem Ecol. 2008 Jul;34(7):928-42. doi: 10.1007/s10886-008-9483-6. Epub 2008 Jun 14. J Chem Ecol. 2008. PMID: 18553119 Review.
-
Recent odor experience selectively modulates olfactory sensitivity across the glomerular output in the mouse olfactory bulb.bioRxiv [Preprint]. 2024 Sep 23:2024.07.21.604478. doi: 10.1101/2024.07.21.604478. bioRxiv. 2024. Update in: Chem Senses. 2025 Jan 22;50:bjae045. doi: 10.1093/chemse/bjae045. PMID: 39386559 Free PMC article. Updated. Preprint.
-
Mimicking biological design and computing principles in artificial olfaction.ACS Chem Neurosci. 2011 May 27;2(9):487-499. doi: 10.1021/cn200027r. ACS Chem Neurosci. 2011. PMID: 22081790 Free PMC article.
-
Neuroethology of Olfactory-Guided Behavior and Its Potential Application in the Control of Harmful Insects.Front Physiol. 2016 Jun 30;7:271. doi: 10.3389/fphys.2016.00271. eCollection 2016. Front Physiol. 2016. PMID: 27445858 Free PMC article. Review.
References
-
- Araneda RC, Kini AD, Firestein S. The molecular receptive range of an odorant receptor. Nat Neurosci. 2000;3:1248–1255. - PubMed
-
- Arbas EA, Humphreys CJ, Ache BW. Morphology and physiological properties of interneurons in the olfactory midbrain of the crayfish. J Comp Physiol [A] 1988;164:231–241. - PubMed
-
- Cromarty SI, Derby CD. Inhibitory receptor binding events among the components of complex mixtures contribute to mixture suppression in responses of olfactory receptor neurons of spiny lobsters. J Comp Physiol [A] 1998;183:699–707. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

