Population pharmacokinetics of rifapentine and its primary desacetyl metabolite in South African tuberculosis patients
- PMID: 16251279
- PMCID: PMC1280164
- DOI: 10.1128/AAC.49.11.4429-4436.2005
Population pharmacokinetics of rifapentine and its primary desacetyl metabolite in South African tuberculosis patients
Abstract
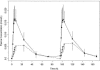
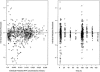
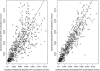
This study was designed to describe the population pharmacokinetics of rifapentine (RFP) and 25-desacetyl RFP in a South African pulmonary tuberculosis patient population. Special reference was made to studying the influence of previous exposure to rifampin (RIF) and the variability in pharmacokinetic parameters between patients and between occasions and the influence of different covariates. Patients were included in the study if they had been receiving first-line antimycobacterial therapy (rifampin, isoniazid, pyrazinamide, and ethambutol) for not less than 4 weeks and not more than 6 weeks and were divided into three RFP dosage groups based on weight: 600 mg, <45 kg; 750 mg, 46 to 55 kg; and 900 mg, >55 kg. Participants received a single oral dose of RFP together with concomitant antimycobacterial agents, excluding RIF, on study days 1 and 5 after they ingested a soup-based meal. The RFP and 25-desacetyl RFP concentration-time data were analyzed by nonlinear mixed-effect modeling using NONMEM. The pharmacokinetics of the parent drug were modeled separately, and the individual pharmacokinetic parameters were used as inputs for the 25-desacetyl RFP pharmacokinetic model. A one-compartment disposition model was found to best describe the data for both the parent and the metabolite, and the metabolite was assumed to be formed only from the central compartment of the parent drug. Prior treatment with RIF did not alter the pharmacokinetics of RFP but appeared to increase the excretion of 25-desacetyl RFP in a nonlinear fashion. The RFP oral clearance and volume of distribution were found to increase by 0.049 liter/h and 0.691 liter, respectively, with a 1-kg increase from the median weight of 50 kg. The oral clearance of 25-desacetyl RFP was found to be 35% lower in female patients. The model developed here describes the population pharmacokinetics of RFP and its primary metabolite in tuberculosis patients and includes the effects of prior administration with RIF and covariate factors.
Figures

Similar articles
-
Consecutive-dose pharmacokinetics of rifapentine in patients diagnosed with pulmonary tuberculosis.Int J Tuberc Lung Dis. 2004 Jul;8(7):862-7. Int J Tuberc Lung Dis. 2004. PMID: 15260278 Clinical Trial.
-
Pharmacokinetics of rifapentine at 600, 900, and 1,200 mg during once-weekly tuberculosis therapy.Am J Respir Crit Care Med. 2004 Jun 1;169(11):1191-7. doi: 10.1164/rccm.200311-1612OC. Epub 2004 Feb 12. Am J Respir Crit Care Med. 2004. PMID: 14962821 Clinical Trial.
-
The early bactericidal activities of rifampin and rifapentine in pulmonary tuberculosis.Am J Respir Crit Care Med. 2005 Jul 1;172(1):128-35. doi: 10.1164/rccm.200411-1557OC. Epub 2005 Apr 1. Am J Respir Crit Care Med. 2005. PMID: 15805182 Clinical Trial.
-
Rifapentine Population Pharmacokinetics and Dosing Recommendations for Latent Tuberculosis Infection.Am J Respir Crit Care Med. 2020 Sep 15;202(6):866-877. doi: 10.1164/rccm.201912-2489OC. Am J Respir Crit Care Med. 2020. PMID: 32412342 Free PMC article.
-
Rifapentine: its role in the treatment of tuberculosis.Ann Pharmacother. 1999 Nov;33(11):1203-10. doi: 10.1345/aph.18450. Ann Pharmacother. 1999. PMID: 10573321 Review.
Cited by
-
Population Pharmacokinetic Modeling and Simulation of Rifapentine Supports Concomitant Antiretroviral Therapy with Efavirenz and Non-Weight Based Dosing.Antimicrob Agents Chemother. 2022 Sep 20;66(9):e0238521. doi: 10.1128/aac.02385-21. Epub 2022 Aug 9. Antimicrob Agents Chemother. 2022. PMID: 35943252 Free PMC article. Clinical Trial.
-
Effects of four different meal types on the population pharmacokinetics of single-dose rifapentine in healthy male volunteers.Antimicrob Agents Chemother. 2010 Aug;54(8):3390-4. doi: 10.1128/AAC.00345-10. Epub 2010 Jun 1. Antimicrob Agents Chemother. 2010. PMID: 20516273 Free PMC article. Clinical Trial.
-
Comparative study on the bioavailability and bioequivalence of rifapentine capsules in humans.Front Pharmacol. 2025 Jan 17;15:1463575. doi: 10.3389/fphar.2024.1463575. eCollection 2024. Front Pharmacol. 2025. PMID: 39898756 Free PMC article.
-
Exploring population pharmacokinetic modeling with resampling visualization.Biomed Res Int. 2014;2014:585687. doi: 10.1155/2014/585687. Epub 2014 May 4. Biomed Res Int. 2014. PMID: 24877118 Free PMC article.
-
Safety and pharmacokinetics of escalating daily doses of the antituberculosis drug rifapentine in healthy volunteers.Clin Pharmacol Ther. 2012 May;91(5):881-8. doi: 10.1038/clpt.2011.323. Clin Pharmacol Ther. 2012. PMID: 22472995 Free PMC article. Clinical Trial.
References
-
- Acocella, G. 1978. Clinical pharmacokinetics of rifampicin. Clin. Pharmacokinet. 3:108-127. - PubMed
-
- American Thoracic Society, Centers for Disease Control and Prevention, and Infectious Diseases Society of America. 2003. Official joint statement on the treatment of tuberculosis. Am. J. Respir. Crit. Care Med. 167:603-662. - PubMed
-
- Aventis Pharmaceuticals. 2000. Priftin package insert. Aventis Pharmaceuticals, Midrand, South Africa.
-
- Battaglia, R., E. Pianezzola, G. Salgarollo, G. Zini, and B. M. Strolin. 1990. Absorption, disposition and preliminary metabolic pathway of 14C-rifabutin in animals and man. J. Antimicrob. Chemother. 26:813-822. - PubMed
-
- Ette, E. I. 1997. Stability and performance of a population pharmacokinetic model. J. Clin. Pharmacol. 37:486-495. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

