Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses
- PMID: 16251287
- PMCID: PMC1280128
- DOI: 10.1128/AAC.49.11.4492-4499.2005
Relationship of pleconaril susceptibility and clinical outcomes in treatment of common colds caused by rhinoviruses
Abstract
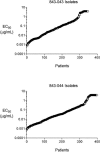
Pleconaril, a specific inhibitor of human picornaviruses, showed therapeutic efficacy against community-acquired colds caused by rhinoviruses in two placebo-controlled trials. Virological assessments were conducted during these trails, including virus culture and drug susceptibility testing. Nasal mucus samples collected from the enrolled patients were tested for the presence of picornavirus by reverse transcriptase PCR and culture. In total, 827 baseline nasal mucus samples were positive by virus culture (420 in the placebo group and 407 in the pleconaril group). Pleconaril treatment was associated with a more rapid loss of culturable virus. By study day 3, the number of samples positive by culture fell to 282 for the placebo-treated subjects and 202 for the pleconaril-treated subjects (P < 0.0001); and by day 6, the number of samples in the two groups positive by culture fell to 196 and 165, respectively (P = 0.07). The clinical benefit correlated strongly with the pleconaril susceptibility of the baseline virus isolate. Pleconaril-treated subjects infected with the more highly susceptible viruses (50% effective concentration < or = 0.38 microg/ml) experienced a median 1.9- to 3.9-day reduction in symptom duration compared with that for the placebo-treated subjects. By contrast, subjects whose baseline virus isolate susceptibility was >0.38 microg/ml did not benefit from pleconaril treatment. These results indicate that the magnitude of symptomatic improvement in pleconaril-treated subjects with community-acquired colds is related to the drug susceptibility of the infecting virus, clearly linking the antiviral effects of the drug to clinical efficacy. Post-baseline virus isolates with reduced susceptibility or full resistance to pleconaril were recovered from 10.7% and 2.7% of drug-treated subjects, respectively. These patients shed low levels of virus and had no unusual clinical outcomes. Nevertheless, studies on the biologic properties and transmissibility of these variant viruses are warranted.
Figures
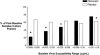

References
-
- Ahmad, A. L., A. B. Dowsett, and D. A. Tyrrell. 1987. Studies of rhinovirus resistant to an antiviral chalcone. Antivir. Res. 8:27-39. - PubMed
-
- Arruda, E., and F. G. Hayden. 1993. Detection of human rhinovirus RNA in nasal washings by PCR. Mol. Cell. Probes 7:373-379. - PubMed
-
- Buchman, C. A., W. J. Doyle, D. Skoner, P. Fireman, and J. M. Gwaltney. 1994. Otologic manifestations of experimental rhinovirus infection. Laryngoscope 104:1295-1299. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

