Identification of a dithiazoline inhibitor of Escherichia coli L,D-carboxypeptidase A
- PMID: 16251288
- PMCID: PMC1280138
- DOI: 10.1128/AAC.49.11.4500-4507.2005
Identification of a dithiazoline inhibitor of Escherichia coli L,D-carboxypeptidase A
Abstract
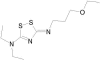
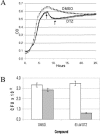
The enzyme L,D-carboxypeptidase A is involved in the recycling of bacterial peptidoglycan and is essential in Escherichia coli during stationary phase. By high-throughput screening, we have identified a dithiazoline inhibitor of the enzyme with a 50% inhibitory concentration of 3 microM. The inhibitor appeared to cause lysis of E. coli during stationary phase, behavior that is similar to a previously described deletion mutant of L,D-carboxypeptidase A (M. F. Templin, A. Ursinus, and J.-V. Holtje, EMBO J. 18:4108-4117, 1999). As much as a one-log drop in CFU in stationary phase was observed upon treatment of E. coli with the inhibitor, and the amount of intracellular tetrapeptide substrate increased by approximately 33%, consistent with inhibition of the enzyme within bacterial cells. Stationary-phase targets such as L,D-carboxypeptidase A are largely underrepresented as targets of the antibiotic armamentarium but provide potential opportunities to interfere with bacterial growth and persistence.
Figures



References
-
- Anderson, M. S., S. S. Eveland, H. R. Onishi, and D. L. Pompliano. 1996. Kinetic mechanism of the Escherichia coli UDPMurNAc-tripeptide d-alanyl-d-alanine-adding enzyme: use of a glutathione S-transferase fusion. Biochemistry 35:16264-16269. - PubMed
-
- Blattner, F. R., G. Plunkett 3rd, C. A. Bloch, N. T. Perna, V. Burland, M. Riley, J. Collado-Vides, J. D. Glasner, C. K. Rode, G. F. Mayhew, J. Gregor, N. W. Davis, H. A. Kirkpatrick, M. A. Goeden, D. J. Rose, B. Mau, and Y. Shao. 1997. The complete genome sequence of Escherichia coli K-12. Science 277:1453-1474. - PubMed
-
- Coates, A., Y. Hu, R. Bax, and C. Page. 2002. The future challenges facing the development of new antimicrobial drugs. Nat. Rev. Drug Disc. 1:895-910. - PubMed
-
- Gutheil, W. G., M. E. Stefanova, and R. A. Nicholas. 2000. Fluorescent coupled enzyme assays for d-alanine: application to penicillin-binding protein and vancomycin activity assays. Anal. Biochem. 287:196-202. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials

