Randomized, placebo-controlled trial of nonpegylated and pegylated forms of recombinant human alpha interferon 2a for suppression of dengue virus viremia in rhesus monkeys
- PMID: 16251289
- PMCID: PMC1280153
- DOI: 10.1128/AAC.49.11.4508-4514.2005
Randomized, placebo-controlled trial of nonpegylated and pegylated forms of recombinant human alpha interferon 2a for suppression of dengue virus viremia in rhesus monkeys
Abstract
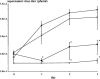
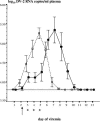
Dengue fever and dengue hemorrhagic fever are caused by infection with any one of the four dengue viruses (DVs) and are significant public health burdens throughout the tropics. Higher viremia levels are associated with greater dengue disease severity. A therapeutic intervention to suppress viremia early in DV infection could potentially ameliorate severe disease. Recombinant alpha interferon 2a (rIFN-alpha-2a, Roferon-A) suppressed DV replication in human peripheral blood mononuclear cells in vitro. We therefore examined the effects of rIFN-alpha-2a and pegylated recombinant IFN-alpha-2a (PEG-rIFN-alpha-2a, PEGASYS) on DV serotype 2 (DV-2) viremia in rhesus monkeys. Flavivirus-naïve monkeys were inoculated with DV-2 and randomized to receive a single dose of rIFN-alpha-2a (10 million international units/m2) versus placebo or PEG-rIFN-alpha-2a (6 microg/kg) versus placebo 1 day after the onset of viremia. Serial daily viremia levels were measured, and convalescent-phase DV-2 neutralizing antibody titers were determined. Compared to placebo, a single injection of rIFN-alpha-2a temporarily suppressed DV-2 replication and delayed the time to peak viremia by a median of 3 days. However, measures of total viral burden were not different between the two groups. A single injection of PEG-rIFN-alpha-2a significantly lowered daily viremia levels and improved virus clearance, starting 48 h after administration. There were no significant differences in DV-2 neutralizing antibody titers between the treatment and placebo groups at 30 and 90 days postinfection. Based on their individual effects, future studies should investigate a combination of rIFN-alpha-2a and PEG-rIFN-alpha-2a for suppression of dengue virus viremia and as a potential therapeutic intervention.
Figures

References
-
- Anonymous. 2004. Physicians' desk reference. Medical Economics, Montvale, N.J.
-
- Calabrese, L. H., M. M. Lederman, J. Spritzler, R. W. Coombs, L. Fox, B. Schock, B. Yen-Lieberman, R. Johnson, D. Mildvan, and N. Parekh. 2002. Placebo-controlled trial of cyclosporin-A in HIV-1 disease: implications for solid organ transplantation. J. Acquir. Immune Defic. Syndr. 29:356-362. - PubMed
-
- Chapuis, A. G., G. Paolo Rizzardi, C. D'Agostino, A. Attinger, C. Knabenhans, S. Fleury, H. Acha-Orbea, and G. Pantaleo. 2000. Effects of mycophenolic acid on human immunodeficiency virus infection in vitro and in vivo. Nat. Med. 6:762-768. - PubMed
-
- De Clercq, E., L. Naesens, L. De Bolle, D. Schols, Y. Zhang, and J. Neyts. 2001. Antiviral agents active against human herpesviruses HHV-6, HHV-7 and HHV-8. Rev. Med. Virol. 11:381-395. - PubMed
-
- DeRoeck, D., J. Deen, and J. D. Clemens. 2003. Policymakers' views on dengue fever/dengue haemorrhagic fever and the need for dengue vaccines in four southeast Asian countries. Vaccine 22:121-129. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

