Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines
- PMID: 16251296
- PMCID: PMC1280114
- DOI: 10.1128/AAC.49.11.4561-4566.2005
Antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines
Abstract
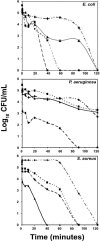
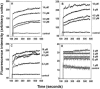
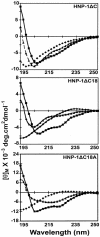
The antibacterial activity of human neutrophil defensin HNP-1 analogs without cysteines has been investigated. A peptide corresponding to the HNP-1 sequence without the six cysteines (HNP-1deltaC) exhibited antibacterial activity toward gram-negative and gram-positive bacteria. Truncated analogs wherein the nine N-terminal residues of HNP-1 and the remaining three cysteines were deleted (HNP-1deltaC18) or the G was replaced with A (HNP-1deltaC18A) also exhibited antibacterial activity. Substantial activity was observed for HNP-1deltaC and HNP-1deltaC18 in the presence of 100 mM NaCl, except in the case of Pseudomonas aeruginosa. The linear peptides were active in the presence of carbonyl cyanide m-chlorophenylhydrazone (CCCP), indicating that proton motive force was not essential for killing of bacteria by the peptides. In fact, in the presence of CCCP, the peptides were active against P. aeruginosa even in the presence of 100 mM NaCl. The antibacterial activity of HNP-1deltaC, but not that of the shorter, 18-residue peptides, was attenuated in the presence of serum. The generation of defensins without cysteines would be easier than that of disulfide-linked defensins. Hence, linear defensins could have potential as therapeutic agents.
Figures
References
-
- Atherton, E., and R. C. Sheppard. 1989. Solid phase peptide synthesis: a practical approach. IRL Press, Oxford, United Kingdom.
-
- Befus, A. D., C. Mowat, M. Gilchrist, J. Hu, S. Solomon, and A. Bateman. 1999. Neutrophil defensins induce histamine secretion from mast cells: mechanisms of action. J. Immunol. 163:947-953. - PubMed
-
- Blanco, F. J., and L. Serrano. 1995. Folding of protein G B1 domain studied by the conformational characterization of fragments comprising its secondary structure elements. Eur. J. Biochem. 230:634-649. - PubMed
-
- Chertov, O., D. F. Michiel, L. Xu, J. M. Wang, K. Tani, W. J. Murphy, D. L. Longo, D. D. Taub, and J. J. Oppenheim. 1996. Identification of defensin-1, defensin-2, and CAP37/azurocidin as T-cell chemoattractant proteins released from interleukin-8-stimulated neutrophils. J. Biol. Chem. 271:2935-2940. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

