Nystatin biosynthesis and transport: nysH and nysG genes encoding a putative ABC transporter system in Streptomyces noursei ATCC 11455 are required for efficient conversion of 10-deoxynystatin to nystatin
- PMID: 16251298
- PMCID: PMC1280151
- DOI: 10.1128/AAC.49.11.4576-4583.2005
Nystatin biosynthesis and transport: nysH and nysG genes encoding a putative ABC transporter system in Streptomyces noursei ATCC 11455 are required for efficient conversion of 10-deoxynystatin to nystatin
Abstract
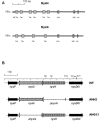
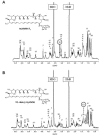
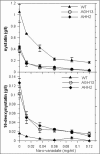
The genes nysH and nysG, encoding putative ABC-type transporter proteins, are located at the flank of the nystatin biosynthetic gene cluster in Streptomyces noursei ATCC 11455. To assess the possible roles of these genes in nystatin biosynthesis, they were inactivated by gene replacements leading to in-frame deletions. Metabolite profile analysis of the nysH and nysG deletion mutants revealed that both of them synthesized nystatin at a reduced level and produced considerable amounts of a putative nystatin analogue. Liquid chromatography-mass spectrometry and nuclear magnetic resonance structural analyses of the latter metabolite confirmed its identity as 10-deoxynystatin, a nystatin precursor lacking a hydroxyl group at C-10. Washing experiments demonstrated that both nystatin and 10-deoxynystatin are transported out of cells, suggesting the existence of an alternative efflux system(s) for the transport of nystatin-related metabolites. This notion was further corroborated in experiments with the ATPase inhibitor sodium o-vanadate, which affected the production of nystatin and 10-deoxynystatin in the wild-type strain and transporter mutants in a different manner. The data obtained in this study suggest that the efflux of nystatin-related polyene macrolides occurs through several transporters and that the NysH-NysG efflux system provides conditions favorable for C-10 hydroxylation.
Figures


Similar articles
-
Characterization of the P450 monooxygenase NysL, responsible for C-10 hydroxylation during biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei.Appl Environ Microbiol. 2006 Apr;72(4):2514-9. doi: 10.1128/AEM.72.4.2514-2519.2006. Appl Environ Microbiol. 2006. PMID: 16597951 Free PMC article.
-
Identification of a gene cluster for antibacterial polyketide-derived antibiotic biosynthesis in the nystatin producer Streptomyces noursei ATCC 11455.Microbiology (Reading). 2000 Mar;146 ( Pt 3):611-619. doi: 10.1099/00221287-146-3-611. Microbiology (Reading). 2000. PMID: 10746764
-
In vivo analysis of the regulatory genes in the nystatin biosynthetic gene cluster of Streptomyces noursei ATCC 11455 reveals their differential control over antibiotic biosynthesis.J Bacteriol. 2004 Mar;186(5):1345-54. doi: 10.1128/JB.186.5.1345-1354.2004. J Bacteriol. 2004. PMID: 14973031 Free PMC article.
-
Biosynthesis of the polyene antifungal antibiotic nystatin in Streptomyces noursei ATCC 11455: analysis of the gene cluster and deduction of the biosynthetic pathway.Chem Biol. 2000 Jun;7(6):395-403. doi: 10.1016/s1074-5521(00)00120-4. Chem Biol. 2000. PMID: 10873841
-
Biosynthesis of the polyene macrolide antibiotic nystatin in Streptomyces noursei.Appl Microbiol Biotechnol. 2005 Jun;67(4):436-43. doi: 10.1007/s00253-004-1802-4. Epub 2005 Feb 8. Appl Microbiol Biotechnol. 2005. PMID: 15700127 Review.
Cited by
-
Corrigendum: The Application of Regulatory Cascades in Streptomyces: Yield Enhancement and Metabolite Mining.Front Microbiol. 2021 Feb 3;11:614274. doi: 10.3389/fmicb.2020.614274. eCollection 2020. Front Microbiol. 2021. PMID: 33613466 Free PMC article.
-
Initiation of polyene macrolide biosynthesis: interplay between polyketide synthase domains and modules as revealed via domain swapping, mutagenesis, and heterologous complementation.Appl Environ Microbiol. 2011 Oct;77(19):6982-90. doi: 10.1128/AEM.05781-11. Epub 2011 Aug 5. Appl Environ Microbiol. 2011. PMID: 21821762 Free PMC article.
-
The Application of Regulatory Cascades in Streptomyces: Yield Enhancement and Metabolite Mining.Front Microbiol. 2020 Mar 24;11:406. doi: 10.3389/fmicb.2020.00406. eCollection 2020. Front Microbiol. 2020. PMID: 32265866 Free PMC article. Review.
-
ABC transporter genes from Streptomyces ghanaensis moenomycin biosynthetic gene cluster: roles in antibiotic production and export.Arch Microbiol. 2012 Nov;194(11):915-22. doi: 10.1007/s00203-012-0827-9. Epub 2012 Jun 21. Arch Microbiol. 2012. PMID: 22717951 Free PMC article.
-
Actinomycete-Derived Polyketides as a Source of Antibiotics and Lead Structures for the Development of New Antimicrobial Drugs.Antibiotics (Basel). 2019 Sep 20;8(4):157. doi: 10.3390/antibiotics8040157. Antibiotics (Basel). 2019. PMID: 31547063 Free PMC article. Review.
References
-
- Aparicio, J. F., R. Fouces, M. V. Mendes, N. Olivera, and J. F. Martín. 2000. A complex multienzyme system encoded by five polyketide synthase genes is involved in the biosynthesis of the 26-membered polyene macrolide pimaricin in Streptomyces natalensis. Chem. Biol. 7:895-905. - PubMed
-
- Aparicio, J. F., P. Caffrey, J. A. Gil, and S. B. Zotchev. 2003. Polyene antibiotic biosynthesis gene clusters. Appl. Microbiol. Biotechnol. 61:179-188. - PubMed
-
- Aszalos, A., A. Bax, N. Burlinson, P. Roller, and C. McNeal. 1985. Physico-chemical and microbiological comparison of nystatin, amphotericin A and amphotericin B, and structure of amphotericin A. J. Antibiot. 38:1699-1713. - PubMed
-
- Bentley, S. D., K. F. Chater, A. M. Cerdeno-Tarraga, G. L. Challis, N. R. Thomson, K. D. James, D. E. Harris, M. A. Quail, H. Kieser, D. Harper, A. Bateman, S. Brown, G. Chandra, C. W. Chen, M. Collins, A. Cronin, A. Fraser, A. Goble, J. Hidalgo, T. Hornsby, S. Howarth, C. H. Huang, T. Kieser, L. Larke, L. Murphy, K. Oliver, S. O'Neil, E. Rabbinowitsch, M. A. Rajandream, K. Rutherford, S. Rutter, K. Seeger, D. Saunders, S. Sharp, R. Squares, S. Squares, K. Taylor, T. Warren, A. Wietzorrek, J. Woodward, B. G. Barrell, J. Parkhill, and D. A. Hopwood. 2002. Complete genome sequence of the model actinomycete Streptomyces coelicolor A3(2). Nature 417:141-147. - PubMed
-
- Bolard, J. 1986. How do the polyene macrolide antibiotics affect the cellular membrane properties? Biochim. Biophys. Acta 864:257-304. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

