Horizontal transfer of iturin A operon, itu, to Bacillus subtilis 168 and conversion into an iturin A producer
- PMID: 16251307
- PMCID: PMC1280175
- DOI: 10.1128/AAC.49.11.4641-4648.2005
Horizontal transfer of iturin A operon, itu, to Bacillus subtilis 168 and conversion into an iturin A producer
Abstract
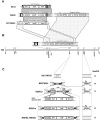
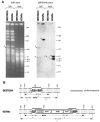
Iturin A and its derivatives are lipopeptide antibiotics produced by Bacillus subtilis and several closely related bacteria. Three iturin group operons (i.e., iturin A, mycosubtilin, and bacillomycin D) of those antibiotic-producing strains have been cloned and sequenced thus far, strongly implying the horizontal transfer of these operons. To examine the nature of such horizontal transfer in terms of antibiotic production, a 42-kb region of the B. subtilis RB14 genome, which contains a complete 38-kb iturin A operon, was transferred via competent cell transformation to the genome of a non-iturin A producer, B. subtilis 168, using a method based on double-crossover homologous recombination with two short landing pad sequences (LPSs) in the genome. The recombinant was positively selected by confirming the elimination of the cI repressor gene, which was localized between the two LPSs and substituted by the transferred segment. The iturin A operon-transferred strain 168 was then converted into an iturin A producer by the introduction of an sfp gene, which encodes 4'-phosphopantetheinyl transferase and is mutated in strain 168. By inserting the pleiotropic regulator degQ, the productivity of iturin A increased sevenfold and was restored to about half that of the donor strain RB14, without the transfer of additional genes, such as regulatory or self-resistance genes.
Figures

Similar articles
-
Cloning, sequencing, and characterization of the iturin A operon.J Bacteriol. 2001 Nov;183(21):6265-73. doi: 10.1128/JB.183.21.6265-6273.2001. J Bacteriol. 2001. PMID: 11591669 Free PMC article.
-
Cloning, sequencing, and characterization of the genetic region relevant to biosynthesis of the lipopeptides iturin A and surfactin in Bacillus subtilis.Curr Microbiol. 2003 Oct;47(4):272-7. doi: 10.1007/s00284-002-4008-y. Curr Microbiol. 2003. PMID: 14629006
-
Biofilm fermentation of iturin A by a recombinant strain of Bacillus subtilis 168.J Biotechnol. 2007 Jan 10;127(3):503-7. doi: 10.1016/j.jbiotec.2006.07.013. Epub 2006 Jul 27. J Biotechnol. 2007. PMID: 16942812
-
Molecular characterization and analysis of the operon encoding the antifungal lipopeptide bacillomycin D.FEMS Microbiol Lett. 2004 May 1;234(1):43-9. doi: 10.1016/j.femsle.2004.03.011. FEMS Microbiol Lett. 2004. PMID: 15109718
-
[Biotechnological Potential of the Bacillus subtilis 20 Strain].Mol Biol (Mosk). 2020 Jan-Feb;54(1):137-145. doi: 10.31857/S0026898420010085. Mol Biol (Mosk). 2020. PMID: 32163397 Review. Russian.
Cited by
-
Purification of Bioactive Lipopeptides Produced by Bacillus subtilis Strain BIA.Chromatographia. 2016;79(21):1527-1532. doi: 10.1007/s10337-016-3164-3. Epub 2016 Sep 17. Chromatographia. 2016. PMID: 27867207 Free PMC article.
-
Development of an efficient electroporation method for iturin A-producing Bacillus subtilis ZK.Int J Mol Sci. 2015 Apr 1;16(4):7334-51. doi: 10.3390/ijms16047334. Int J Mol Sci. 2015. PMID: 25837631 Free PMC article.
-
Identification of Lipopeptide Iturin A Produced by Bacillus amyloliquefaciens NCPSJ7 and Its Antifungal Activities against Fusarium oxysporum f. sp. niveum.Foods. 2022 Sep 26;11(19):2996. doi: 10.3390/foods11192996. Foods. 2022. PMID: 36230072 Free PMC article.
-
Iturin: A Promising Cyclic Lipopeptide with Diverse Applications.Biomolecules. 2023 Oct 12;13(10):1515. doi: 10.3390/biom13101515. Biomolecules. 2023. PMID: 37892197 Free PMC article. Review.
-
Organization and characterization of genetic regions in Bacillus subtilis subsp. krictiensis ATCC55079 associated with the biosynthesis of iturin and surfactin compounds.PLoS One. 2017 Dec 21;12(12):e0188179. doi: 10.1371/journal.pone.0188179. eCollection 2017. PLoS One. 2017. PMID: 29267290 Free PMC article.
References
-
- Aharonowitz, Y., G. Cohen, and J. F. Martin. 1992. Penicillin and cephalosporin biosynthetic genes: structure, organization, regulation, and evolution. Annu. Rev. Microbiol. 46:461-495. - PubMed
-
- Duitman, E. H., L. W. Hamoen, M. Rembold, G. Venema, H. Seitz, W. Saenger, F. Bernhard, R. Reinhardt, M. Schmidt, C. Ullrich, T. Stein, F. Leenders, and J. Vater. 1999. The mycosubtilin synthetase of Bacillus subtilis ATCC6633: a multifunctional hybrid between a peptide synthetase, an amino transferase, and a fatty acid synthase. Proc. Natl. Acad. Sci. USA 96:13294-13299. - PMC - PubMed
-
- Eppelmann, K., S. Doekel, and M. A. Marahiel. 2001. Engineered biosynthesis of the peptide antibiotic bacitracin in the surrogate host Bacillus subtilis. J. Biol. Chem. 276:34824-34831. - PubMed
-
- Guenzi, E., G. Galli, I. Grgurina, E. Pace, P. Ferranti, and G. Grandi. 1998. Coordinate transcription and physical linkage of domains in surfactin synthetase are not essential for proper assembly and activity of the multienzyme complex. J. Biol. Chem. 273:14403-14410. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

