Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam
- PMID: 16251309
- PMCID: PMC1280174
- DOI: 10.1128/AAC.49.11.4658-4666.2005
Safety and efficacy of tigecycline in treatment of skin and skin structure infections: results of a double-blind phase 3 comparison study with vancomycin-aztreonam
Abstract
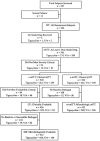
In a randomized, double-blind, controlled trial, 546 patients with complicated skin and skin structure infections received tigecycline 100 mg/day (a 100-mg initial dose and then 50 mg intravenously twice daily) or the combination of vancomycin 2 g/day (1 g intravenously twice daily) and aztreonam 4 g/day (2 g intravenously twice daily) for up to 14 days. The primary end point was the clinical response in the clinical modified intent-to-treat (c-mITT) and clinically evaluable (CE) populations at the test-of-cure visit 12 to 92 days after the last dose. The microbiologic response at the test-of-cure visit was also assessed. Safety was assessed by physical examination, laboratory results, and adverse event reporting. Five hundred twenty patients were included in the c-mITT population (tigecycline group, n = 261; combination group, n = 259), and 436 were clinically evaluable (tigecycline group, n = 223; combination group, n = 213). The clinical responses in the tigecycline and the combination vancomycin and aztreonam groups were similar in the c-mITT population (84.3% versus 86.9%; difference, -2.6% [95% confidence interval, -9.0, 3.8]; P = 0.4755) and the CE population (89.7% versus 94.4%; difference, -4.7% [95% confidence interval, -10.2, 0.8]; P = 0.1015). Microbiologic eradication (documented or presumed) occurred in 84.8% of the patients receiving tigecycline and 93.2% of the patients receiving vancomycin and aztreonam (difference, -8.5 [95% confidence interval, -16.0, -1.0]; P = 0.0243). The numbers of patients reporting adverse events were similar in the two groups, with increased nausea and vomiting rates in the tigecycline group and an increased incidence of rash and increases in alanine aminotransferase and aspartate aminotransferase levels in the combination vancomycin and aztreonam group. Tigecycline was shown to be safe and effective for the treatment of complicated skin and skin structure infections.
References
-
- Betriu, C., E. Culebras, I. Rodriguez-Avial, M. Gomez, B. A. Sanchez, and J. J. Picazo. 2004. In vitro activities of tigecycline against erythromycin-resistant Streptococcus pyogenes and Streptococcus agalactiae: mechanisms of macrolide and tetracycline resistance. Antimicrob. Agents Chemother. 48:323-325. - PMC - PubMed
-
- Biedenbach, D. J., M. L. Beach, and R. N. Jones. 2001. In vitro antimicrobial activity of GAR-936 tested against antibiotic-resistant gram-positive blood stream infection isolates and strains producing extended-spectrum beta-lactamases. Diagn. Microbiol. Infect. Dis. 40:173-177. - PubMed
-
- Boyce, J. M. 1992. Methicillin-resistant Staphylococcus aureus in hospitals and long-term care facilities: microbiology, epidemiology, and preventive measures. Infect. Control Hosp. Epidemiol. 13:725-737. - PubMed
-
- Brook, I., and E. H. Frazier. 1990. Aerobic and anaerobic bacteriology of wounds and cutaneous abscesses. Arch. Surg. 125:1445-1451. - PubMed
-
- Centers for Disease Control and Prevention. 2002. Staphylococcus aureus resistant to vancomycin—United States, 2002. Morb. Mortal. Wkly. Rep. 51:565-567. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources