Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency
- PMID: 16251314
- PMCID: PMC1280119
- DOI: 10.1128/AAC.49.11.4700-4707.2005
Comparative effects of two neutralizing anti-respiratory syncytial virus (RSV) monoclonal antibodies in the RSV murine model: time versus potency
Abstract
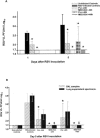
Respiratory syncytial virus (RSV) is the leading viral pathogen responsible for bronchiolitis and pneumonia in infants and young children worldwide. We have previously shown in the mouse model that treatment with an anti-RSV neutralizing monoclonal antibody (MAb) against the F glycoprotein of RSV, palivizumab, decreased lung inflammation, airway obstruction, and postmethacholine airway hyperresponsiveness. MEDI-524, or Numax, is a new MAb derived from palivizumab with enhanced neutralizing activity against RSV. We compared the effects of these two MAbs on different markers of disease severity using the murine model of RSV infection. BALB/c mice were intranasally inoculated with RSV A2. Palivizumab or MEDI-524 was administered once at either 24 h before or 48 h after RSV inoculation. Regardless of the time of administration, all treated mice showed significantly decreased RSV loads in bronchoalveolar lavage samples measured by plaque assay. Only MEDI-524 given at -24 h significantly decreased lung RSV RNA loads on days 5 and 28 after RSV inoculation. Pulmonary histopathologic scores, airway obstruction, and postmethacholine airway hyperresponsiveness were significantly reduced in mice treated with MEDI-524 at 24 h before inoculation, compared with untreated controls and the other regimens evaluated. MEDI-524 was superior to palivizumab on several outcome variables of RSV disease assessed in the mouse model: viral replication, inflammatory and clinical markers of acute disease severity, and long-term pulmonary abnormalities.
Figures


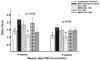
References
-
- 2003. American Academy of Pediatrics. 2003. 2003 report of the Committee on Infectious Diseases, 26th ed. American Academy of Pediatrics, Elk Grove Village, Ill.
-
- Boyce, T. G., B. G. Mellen, E. F. Mitchel, Jr., P. F. Wright, and M. R. Griffin. 2000. Rates of hospitalization for respiratory syncytial virus infection among children in Medicaid. J. Pediatr. 137:865-870. - PubMed
-
- Buckingham, S. C., A. J. Bush, and J. P. Devincenzo. 2000. Nasal quantity of respiratory syncytical virus correlates with disease severity in hospitalized infants. Pediatr. Infect. Dis. J. 19:113-117. - PubMed
-
- Campbell, E. M., S. L. Kunkel, R. M. Strieter, and N. W. Lukacs. 1998. Temporal role of chemokines in a murine model of cockroach allergen-induced airway hyperreactivity and eosinophilia. J. Immunol. 161:7047-7053. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

