Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity
- PMID: 16251317
- PMCID: PMC1280117
- DOI: 10.1128/AAC.49.11.4721-4732.2005
Maraviroc (UK-427,857), a potent, orally bioavailable, and selective small-molecule inhibitor of chemokine receptor CCR5 with broad-spectrum anti-human immunodeficiency virus type 1 activity
Abstract
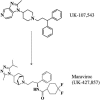
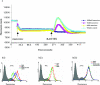
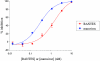
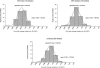
Maraviroc (UK-427,857) is a selective CCR5 antagonist with potent anti-human immunodeficiency virus type 1 (HIV-1) activity and favorable pharmacological properties. Maraviroc is the product of a medicinal chemistry effort initiated following identification of an imidazopyridine CCR5 ligand from a high-throughput screen of the Pfizer compound file. Maraviroc demonstrated potent antiviral activity against all CCR5-tropic HIV-1 viruses tested, including 43 primary isolates from various clades and diverse geographic origin (geometric mean 90% inhibitory concentration of 2.0 nM). Maraviroc was active against 200 clinically derived HIV-1 envelope-recombinant pseudoviruses, 100 of which were derived from viruses resistant to existing drug classes. There was little difference in the sensitivity of the 200 viruses to maraviroc, as illustrated by the biological cutoff in this assay (= geometric mean plus two standard deviations [SD] of 1.7-fold). The mechanism of action of maraviroc was established using cell-based assays, where it blocked binding of viral envelope, gp120, to CCR5 to prevent the membrane fusion events necessary for viral entry. Maraviroc did not affect CCR5 cell surface levels or associated intracellular signaling, confirming it as a functional antagonist of CCR5. Maraviroc has no detectable in vitro cytotoxicity and is highly selective for CCR5, as confirmed against a wide range of receptors and enzymes, including the hERG ion channel (50% inhibitory concentration, >10 microM), indicating potential for an excellent clinical safety profile. Studies in preclinical in vitro and in vivo models predicted maraviroc to have human pharmacokinetics consistent with once- or twice-daily dosing following oral administration. Clinical trials are ongoing to further investigate the potential of using maraviroc for the treatment of HIV-1 infection and AIDS.
Figures

References
-
- Barber, C. G. 2004. CCR5 antagonists for the treatment of HIV. Curr. Opin. Investig. Drugs 5:851-861. - PubMed
-
- Beatty, C., M. Bradley, D. Brambilla, F. Breakenridge, J. W. Bremer, J. Dargavon, B. Ladd, D. Livnatt, C. Michel, C. Mundy, V. Price, T. Ramacciotti, P. Reichelderfer, B. Staes, C. Starkey, and M. Winters. 1997. DAIDS Virology Manual for HIV Laboratories, p. 73-76. Division of Acquired Immunodeficiency Syndrome, NIAID, NIH, Bethesda, Md.
-
- Berger, E. A., P. M. Murphy, and J. M. Farber. 1999. Chemokine receptors as HIV-1 coreceptors: roles in viral entry, tropism, and disease. Annu. Rev. Immunol. 17:657-700. - PubMed
-
- Bradley, J., J. Gill, F. Bertelli, S. Letafat, R. Corbau, P. Hayter, P. Harrison, A. Tee, W. Keighley, M. Perros, G. Ciaramella, A. Sewing, and C. Williams. 2004. Development and automation of a 384-well cell fusion assay to identify inhibitors of CCR5/CD4-mediated HIV virus entry. J. Biomol. Screen 9:516-524. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

