Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis
- PMID: 16251405
- PMCID: PMC1603794
- DOI: 10.1016/S0002-9440(10)61208-4
Origin of interstitial fibroblasts in an accelerated model of angiotensin II-induced renal fibrosis
Abstract
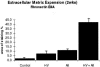
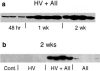
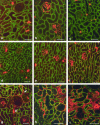
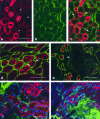
To determine whether previous renal injury accelerates the progression of glomerulosclerosis and interstitial fibrosis, we examined the effect of treating rats with angiotensin II after Habu venom injury. After initiating disease, we examined the origin of interstitial myofibroblasts by locating alpha-smooth muscle actin (alpha-SMA)-positive and Na+,K+-ATPase-positive cells relative to interstitial space, tubular epithelial cells, the tubular basement membrane (TBM), and vascular structures. Tubular epithelial-mesenchymal transition was also assessed by examining TBM integrity and by using Texas Red (TR)-dextran in intravital tracking experiments. The staining of alpha-SMA-positive myofibroblasts dramatically increased in peritubular interstitial spaces 48 hours after Habu venom plus angiotensin II, particularly in and around perivascular and periglomerular regions, while tubular epithelial cells were alpha-SMA-negative. Na+,K+-ATPase-positive and TR-dextran-labeled cells were restricted to the tubular epithelium and excluded from the interstitium. By 7 and 14 days, expanded interstitial space contained only alpha-SMA-positive myofibroblasts without TR-dextran endocytic particles. Epithelium of atrophic tubules containing TR-dextran remained confined by surrounding interstitium and myofibroblasts. These studies indicate that early expansion of alpha-SMA-positive cells in the interstitium and loss of tubular area occur via encroachment of interstitial myofibroblasts from perivascular into atrophic tubular spaces rather than via epithelial-mesenchymal transition and migration of tubular cells through the TBM into the interstitium.
Figures


Similar articles
-
Tubular epithelial-myofibroblast transdifferentiation in progressive tubulointerstitial fibrosis in 5/6 nephrectomized rats.Kidney Int. 1998 Sep;54(3):864-76. doi: 10.1046/j.1523-1755.1998.00076.x. Kidney Int. 1998. PMID: 9734611
-
Actin filaments in human renal tubulo-interstitial fibrosis: significance for the concept of epithelial-myofibroblast transformation.J Submicrosc Cytol Pathol. 2003 Jul;35(3):221-33. J Submicrosc Cytol Pathol. 2003. PMID: 14690170
-
Myofibroblast phenotypes expression in experimental renal scarring.Nephrol Dial Transplant. 1997 May;12(5):904-15. doi: 10.1093/ndt/12.5.904. Nephrol Dial Transplant. 1997. PMID: 9175042
-
The ultrastructure of human tubulo-interstitial fibrosis.J Submicrosc Cytol Pathol. 2003 Apr;35(2):147-60. J Submicrosc Cytol Pathol. 2003. PMID: 12974328 Review.
-
Direct contribution of epithelium to organ fibrosis: epithelial-mesenchymal transition.Hum Pathol. 2009 Oct;40(10):1365-76. doi: 10.1016/j.humpath.2009.02.020. Epub 2009 Aug 19. Hum Pathol. 2009. PMID: 19695676 Review.
Cited by
-
NAD(P)H oxidase mediates TGF-beta1-induced activation of kidney myofibroblasts.J Am Soc Nephrol. 2010 Jan;21(1):93-102. doi: 10.1681/ASN.2009020146. Epub 2009 Nov 19. J Am Soc Nephrol. 2010. PMID: 19926889 Free PMC article.
-
RhoA/Rho kinase mediates TGF-β1-induced kidney myofibroblast activation through Poldip2/Nox4-derived reactive oxygen species.Am J Physiol Renal Physiol. 2014 Jul 15;307(2):F159-71. doi: 10.1152/ajprenal.00546.2013. Epub 2014 May 28. Am J Physiol Renal Physiol. 2014. PMID: 24872317 Free PMC article.
-
Polymorphisms in the 3' UTR in the neurocalcin delta gene affect mRNA stability, and confer susceptibility to diabetic nephropathy.Hum Genet. 2007 Nov;122(3-4):397-407. doi: 10.1007/s00439-007-0414-3. Epub 2007 Aug 2. Hum Genet. 2007. PMID: 17671797
-
Exploring unconventional targets in myofibroblast transdifferentiation outside classical TGF- signaling in renal fibrosis.Front Physiol. 2024 May 14;15:1296504. doi: 10.3389/fphys.2024.1296504. eCollection 2024. Front Physiol. 2024. PMID: 38808357 Free PMC article. Review.
-
mTORC2 Signaling Regulates Nox4-Induced Podocyte Depletion in Diabetes.Antioxid Redox Signal. 2016 Nov 1;25(13):703-719. doi: 10.1089/ars.2015.6562. Epub 2016 Sep 12. Antioxid Redox Signal. 2016. PMID: 27393154 Free PMC article.
References
-
- Border WA, Noble NA. Interactions of transforming growth factor-beta and angiotensin II in renal fibrosis. Hypertension. 1998;31:181–188. - PubMed
-
- Klahr S, Morrissey JJ. Comparative study of ACE inhibitors and angiotensin II receptor antagonists in interstitial scarring. Kidney Int. 1997;52:S111–S114. - PubMed
-
- Wolf G, Neilson EG. Angiotensin II as a renal growth factor. J Am Soc Nephrol. 1993;3:1531–1540. - PubMed
-
- Klahr S, Morrissey JJ. The role of vasoactive compounds, growth factors and cytokines in the progression of renal disease. Kidney Int. 2000;57:S7–S14. - PubMed
-
- Matsusaka T, Hymes J, Ichikawa I. Angiotensin in progressive renal diseases: theory and practice. J Am Soc Nephrol. 1996;7:2025–2043. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

