Conditional ablation of macrophages halts progression of crescentic glomerulonephritis
- PMID: 16251406
- PMCID: PMC1603796
- DOI: 10.1016/S0002-9440(10)61209-6
Conditional ablation of macrophages halts progression of crescentic glomerulonephritis
Abstract
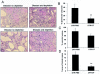
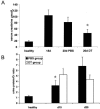
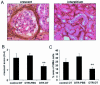
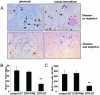
The presence of macrophages in inflamed glomeruli of rat kidney correlates with proliferation and apoptosis of resident glomerular mesangial cells. We assessed the contribution of inflammatory macrophages to progressive renal injury in murine crescentic glomerulonephritis (GN). Using a novel transgenic mouse (CD11b-DTR) in which tissue macrophages can be specifically and selectively ablated by minute injections of diphtheria toxin, we depleted renal inflammatory macrophages through days 15 and 20 of progressive crescentic GN. Macrophage depletion reduced the number of glomerular crescents, improved renal function, and reduced proteinuria. Morphometric analysis of renal tubules and interstitium revealed a marked attenuation of tubular injury that was associated with reduced proliferation and apoptosis of tubular cells. The population of interstitial myofibroblasts decreased after macrophage depletion and interstitial fibrosis also decreased. In the presence of macrophages, interstitial myofibroblasts exhibited increased levels of both proliferation and apoptosis, suggesting that macrophages act to support a population of renal myofibroblasts in a high turnover state and in matrix deposition. Finally, deletion of macrophages reduced CD4 T cells in the diseased kidney. This study demonstrates that macrophages are key effectors of disease progression in crescentic GN, acting to regulate parenchymal cell populations by modulating both cell proliferation and apoptosis.
Figures


References
-
- Lang R, Lustig M, Francois F, Sellinger M, Plesken H. Apoptosis during macrophage-dependent ocular tissue remodelling. Development. 1994;120:3395–3403. - PubMed
-
- Diez-Roux G, Lang RA. Macrophages induce apoptosis in normal cells in vivo. Development. 1997;124:3633–3638. - PubMed
-
- Diez-Roux G, Argilla M, Makarenkova H, Ko K, Lang RA. Macrophages kill capillary cells in G1 phase of the cell cycle during programmed vascular regression. Development. 1999;126:2141–2147. - PubMed
-
- Duffield JS, Erwig LP, Wei X, Liew FY, Rees AJ, Savill JS. Activated macrophages direct apoptosis and suppress mitosis of mesangial cells. J Immunol. 2000;164:2110–2119. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

