Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors
- PMID: 16251412
- PMCID: PMC1603778
- DOI: 10.1016/S0002-9440(10)61215-1
Mouse fetal liver cells in artificial capillary beds in three-dimensional four-compartment bioreactors
Abstract
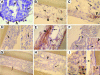
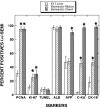
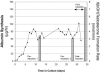
Bioreactors containing porcine or adult human hepatocytes have been used to sustain acute liver failure patients until liver transplantation. However, prolonged function of adult hepatocytes has not been achieved due to compromised proliferation and viability of adult cells in vitro. We investigated the use of fetal hepatocytes as an alternative cell source in bioreactors. Mouse fetal liver cells from gestational day 17 possessed intermediate differentiation and function based on their molecular profile. When cultured in a three-dimensional four-compartment hollow fiber-based bioreactor for 3 to 5 weeks these cells formed neo-tissues that were characterized comprehensively. Albumin liberation, testosterone metabolism, and P450 induction were demonstrated. Histology showed predominant ribbon-like three-dimensional structures composed of hepatocytes between hollow fibers. High positivity for proliferating cell nuclear antigen and Ki-67 and low positivity for terminal dUTP nick-end labeling indicated robust cell proliferation and survival. Most cells within these ribbon arrangements were albumin-positive. In addition, cells in peripheral zones were simultaneously positive for alpha-fetoprotein, cytokeratin-19, and c-kit, indicating their progenitor phenotype. Mesenchymal components including endothelial, stellate, and smooth muscle cells were also observed. Thus, fetal liver cells can survive, proliferate, differentiate, and function in a three-dimensional perfusion culture system while maintaining a progenitor pool, reflecting an important advance in hepatic tissue engineering.
Figures




References
-
- Patzer IJ, Lopez RC, Zhu Y, Wang ZF, Mazariegos GV, Fung JJ. Bioartificial liver assist devices in support of patients with liver failure. Hepatobiliary Pancreat Dis Int. 2002;1:18–25. - PubMed
-
- Gerlach JC. Development of a hybrid liver support system: a review. Int J Artif Organs. 1996;19:645–654. - PubMed
-
- Rudow DL, Russo MW, Hafliger S, Emond JC, Brown RS., Jr Clinical and ethnic differences in candidates listed for liver transplantation with and without potential living donors. Liver Transpl. 2003;9:254–259. - PubMed
-
- Dowling DJ, Mutimer DJ. Artificial liver support in acute liver failure. Eur J Gastroenterol Hepatol. 1999;11:991–996. - PubMed
-
- Monga A, Jain SK. Artificial liver support systems. J Assoc Physicians India. 1999;47:1092–1099. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

