Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases
- PMID: 16251431
- PMCID: PMC6725579
- DOI: 10.1523/JNEUROSCI.3497-05.2005
Alpha7 neuronal nicotinic acetylcholine receptors are negatively regulated by tyrosine phosphorylation and Src-family kinases
Abstract
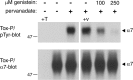
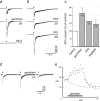
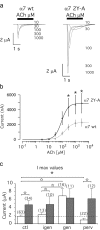
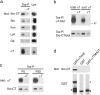
Nicotine, a component of tobacco, is highly addictive but possesses beneficial properties such as cognitive improvements and memory maintenance. Involved in these processes is the neuronal nicotinic acetylcholine receptor (nAChR) alpha7, whose activation triggers depolarization, intracellular signaling cascades, and synaptic plasticity underlying addiction and cognition. It is therefore important to investigate intracellular mechanisms by which a cell regulates alpha7 nAChR activity. We have examined the role of phosphorylation by combining molecular biology, biochemistry, and electrophysiology in SH-SY5Y neuroblastoma cells, Xenopus oocytes, rat hippocampal interneurons, and neurons from the supraoptic nucleus, and we found tyrosine phosphorylation of alpha7 nAChRs. Tyrosine kinase inhibition by genistein decreased alpha7 nAChR phosphorylation but strongly increased acetylcholine-evoked currents, whereas tyrosine phosphatase inhibition by pervanadate produced opposite effects. Src-family kinases (SFKs) directly interacted with the cytoplasmic loop of alpha7 nAChRs and phosphorylated the receptors at the plasma membrane. SFK inhibition by PP2 [4-amino-5-(4-chlorophenyl)-7-(t-butyl)pyrazolo[3,4-d]pyrimidine] or SU6656 (2,3-dihydro-N,N-dimethyl-2-oxo-3-[(4,5,6,7-tetrahydro-1H-indol-2-yl)methylene]-1H-indole-5-sulfonamide) increased alpha7 nAChR-mediated responses, whereas expression of active Src reduced alpha7 nAChR activity. Mutant alpha7 nAChRs lacking cytoplasmic loop tyrosine residues because of alanine replacement of Tyr-386 and Tyr-442 were more active than wild-type receptors and insensitive to kinase or phosphatase inhibition. Because the amount of surface alpha7 receptors was not affected by kinase or phosphatase inhibitors, these data show that functional properties of alpha7 nAChRs depend on the tyrosine phosphorylation status of the receptor and are the result of a balance between SFKs and tyrosine phosphatases. These findings reveal novel regulatory mechanisms that may help to understand nicotinic receptor-dependent plasticity, addiction, and pathology.
Figures






Similar articles
-
Rapid upregulation of alpha7 nicotinic acetylcholine receptors by tyrosine dephosphorylation.J Neurosci. 2005 Apr 6;25(14):3712-23. doi: 10.1523/JNEUROSCI.5389-03.2005. J Neurosci. 2005. PMID: 15814802 Free PMC article.
-
A novel positive allosteric modulator of the alpha7 neuronal nicotinic acetylcholine receptor: in vitro and in vivo characterization.J Neurosci. 2005 Apr 27;25(17):4396-405. doi: 10.1523/JNEUROSCI.5269-04.2005. J Neurosci. 2005. PMID: 15858066 Free PMC article.
-
A novel nicotinic acetylcholine receptor subtype in basal forebrain cholinergic neurons with high sensitivity to amyloid peptides.J Neurosci. 2009 Jan 28;29(4):918-29. doi: 10.1523/JNEUROSCI.3952-08.2009. J Neurosci. 2009. PMID: 19176801 Free PMC article.
-
Regulation of nicotinic acetylcholine receptors by tyrosine kinases in the peripheral and central nervous system: same players, different roles.Cell Mol Life Sci. 2006 Dec;63(23):2818-28. doi: 10.1007/s00018-006-6081-z. Cell Mol Life Sci. 2006. PMID: 17086381 Free PMC article. Review.
-
α7 nicotinic acetylcholine receptors in the hippocampal circuit: taming complexity.Trends Neurosci. 2022 Feb;45(2):145-157. doi: 10.1016/j.tins.2021.11.006. Epub 2021 Dec 13. Trends Neurosci. 2022. PMID: 34916082 Free PMC article. Review.
Cited by
-
Nicotine exposure and the progression of chronic kidney disease: role of the α7-nicotinic acetylcholine receptor.Am J Physiol Renal Physiol. 2012 Jul 15;303(2):F304-12. doi: 10.1152/ajprenal.00661.2011. Epub 2012 May 2. Am J Physiol Renal Physiol. 2012. PMID: 22552933 Free PMC article.
-
Nicotine activates and up-regulates nicotinic acetylcholine receptors in bronchial epithelial cells.Am J Respir Cell Mol Biol. 2009 Jul;41(1):93-9. doi: 10.1165/rcmb.2008-0352OC. Epub 2008 Dec 18. Am J Respir Cell Mol Biol. 2009. PMID: 19097990 Free PMC article.
-
The HSPG syndecan is a core organizer of cholinergic synapses.J Cell Biol. 2021 Sep 6;220(9):e202011144. doi: 10.1083/jcb.202011144. Epub 2021 Jul 2. J Cell Biol. 2021. PMID: 34213535 Free PMC article.
-
Nicotinic Acetylcholine Receptor Alpha7 Subunit Mediates Vagus Nerve Stimulation-Induced Neuroprotection in Acute Permanent Cerebral Ischemia by a7nAchR/JAK2 Pathway.Med Sci Monit. 2017 Dec 23;23:6072-6081. doi: 10.12659/msm.907628. Med Sci Monit. 2017. PMID: 29274273 Free PMC article.
-
Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: advantages and limitations.Biochem Pharmacol. 2011 Oct 15;82(8):915-30. doi: 10.1016/j.bcp.2011.05.001. Epub 2011 May 14. Biochem Pharmacol. 2011. PMID: 21575610 Free PMC article. Review.
References
-
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y (1987) Genistein, a specific inhibitor of tyrosine-specific protein kinases. J Biol Chem 262: 5592-5595. - PubMed
-
- Alkondon M, Reinhardt S, Lobron C, Hermsen B, Maelicke A, Albuquerque EX (1994) Diversity of nicotinic acetylcholine receptors in rat hippocampal neurons. II. The rundown and inward rectification of agonistelicited whole-cell currents and identification of receptor subunits by in situ hybridization. J Pharmacol Exp Ther 271: 494-506. - PubMed
-
- Alkondon M, Pereira EF, Barbosa CT, Albuquerque EX (1997) Neuronal nicotinic acetylcholine receptor activation modulates gammaaminobutyric acid release from CA1 neurons of rat hippocampal slices. J Pharmacol Exp Ther 283: 1396-1411. - PubMed
-
- Alkondon M, Pereira EF, Albuquerque EX (1998) Alpha-bungarotoxin- and methyllycaconitine-sensitive nicotinic receptors mediate fast synaptic transmission in interneurons of rat hippocampal slices. Brain Res 810: 257-263. - PubMed
-
- Balduzzi R, Cupello A, Robello M (2002) Modulation of the expression of GABA(A) receptors in rat cerebellar granule cells by protein tyrosine kinases and protein kinase C. Biochim Biophys Acta 1564: 263-270. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous
